Estrogen
Estrogen and Progesterone: Beyond Reproduction
Neuroscience research explains how reproductive hormones protect the brain.
Posted March 23, 2018 Reviewed by Abigail Fagan
Once again I am extremely lucky to have this article co-written by a senior graduate student who's an expert on how reproductive hormones act on the brain. Stephanie Koebele is completing her Ph.D. studies in Dr. Heather Bimonte-Nelson's neuroscience lab in the Psychology Department at Arizona State University.
Mary is a busy woman. Her son just started college, and her daughter is about to graduate from high school. She works as a pharmacist and volunteers in her community on weekends. While Mary has always had a lot of energy, she has noticed in the past few years that she has been feeling sudden and uncomfortable flushes of heat and feels unusually anxious or sad sometimes. Now at 53, she is noticing her mind doesn’t feel as sharp as it used to – sometimes she misplaces her keys or forgets the grocery list. Mary is worried she might be experiencing some early signs of dementia. But is this the most likely explanation?
Fortunately, not all memory lapses in midlife are signs of future cognitive decline. For the past several years, Mary has actually been undergoing the transition to menopause. Indeed, around age 50, women start to experience several physiological indicators including: hot flashes, night sweats, weight gain, fatigue, mood changes, and memory fog. These undesirable symptoms are all completely normal, common, and most often related to the transition to menopause. Mary has heard that hormone therapy can help with her hot flashes and is considering asking her doctor about her options. She wants to understand all the benefits, risks, and effects these hormones can have on her body.
What Exactly Is Menopause?
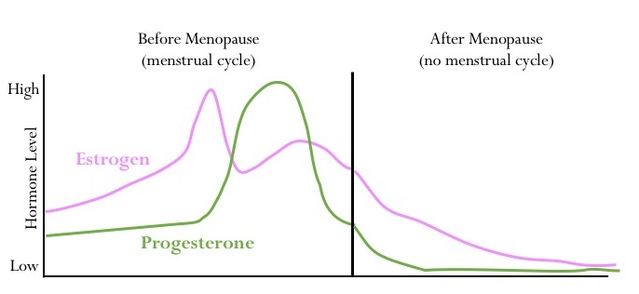
The ovaries are the body’s main production center for the sex steroid hormones estrogen and progesterone. Women are born with all of the eggs they will ever have in their ovaries. Throughout life, some of these eggs grow and are ovulated during each menstrual cycle, during which circulating estrogen levels typically range between 50-300 pg/ml and progesterone between 1-20 ng/ml. Most eggs never mature and will instead be eliminated naturally by a process called atresia.
By the time a woman is in her 50’s, there are very few eggs left in the ovaries, and consequently, there are significantly decreased or even undetectable levels of estrogens and progesterone in the body. After the menopause (defined as 12 consecutive months with no menstrual period), when there are no eggs left, a woman will be considered “post-menopausal.” This phenomenon has gained import as humans are living increasingly longer lives. Thus, a woman may live 30-50 years in this post-menopausal life stage and experience unique changes that can significantly impact her quality of life.
Most women, like Mary, experience a natural transition to menopause over the course of four to ten years; however, 10-15% of women undergo gynecological surgery for a variety of reasons. One such surgery, oophorectomy (surgically removing the ovaries) results in an abrupt drop in ovary-derived hormones like estrogen and progesterone to undetectable levels. This is essentially a surgically-induced menopause that can happen at any point in a woman’s life.
Estrogen and Progesterone
The sex steroid hormones estrogen and progesterone were discovered less than 100 years ago. It was believed that these hormones were only important for reproduction. Estrogen regulates the menstrual cycle and ovulation, controls secondary sex characteristic development during puberty, and helps prepare the reproductive system for potential pregnancy. Progesterone, a “pro-gestational” hormone, works together with estrogen to prepare the uterus for pregnancy, promotes implantation of a fertilized egg into the uterus, and is a crucial element for maintaining a pregnancy. If pregnancy does not occur in a given month, the natural cyclic decrease of progesterone initiates the menstrual period.
Beyond these well-known reproductive functions, more recent discoveries indicate diverse and far-reaching non-reproductive roles for estrogen and progesterone, including beneficial effects on bones, hair, nails, and skin. One surprising discovery is that estrogen and progesterone have potent non-reproductive effects in the brain too.
How do estrogen and progesterone get into the brain and what exactly are these “reproductive hormones” doing there? Sex steroid hormones like estrogen and progesterone are made from cholesterol and travel in the blood. They easily cross through cell membranes, as well as through the blood-brain barrier, which only allows certain molecules to get into the brain. Once inside the central nervous system, the hormones act at receptor targets and trigger changes, such as increasing or decreasing protein and neurotransmitter levels, which significantly affects many brain structures and functions.
Below are four ways these “reproductive hormones” influence the brain outside of their usual roles in reproduction, and why women, like Mary, should care about these effects.
Learning and Memory
Mary’s forgetfulness is a common, though under-recognized, symptom of menopause.
One of the best studied functions of sex steroid hormones is their role in learning and memory. A brain structure called the hippocampus is intimately involved in learning and memory; especially forming explicit memories (i.e. memories for facts and personal experiences).
The hippocampus has many receptor or action sites specifically for estrogen and progesterone. So how does the loss of these hormones with natural or surgical menopause impact brain health? The Neuroscience of Memory and Aging Laboratory at Arizona State University, led by Dr. Heather Bimonte-Nelson, seeks to answer questions about the effects and roles of these hormones on the brain.
Studying the effects of ovary-derived hormones on the human brain is challenging. Scientists often rely on animal models (typically rodents, which have a similar reproductive system) of human menopause. Two prominent rodent models of menopause include a surgical model, called ovariectomy (surgical ovary removal), and a transitional menopause model, using a drug called VCD.
The Bimonte-Nelson lab showed that surgical ovary removal in rats was associated with poorer spatial working memory; a type of short-term memory that requires remembering several pieces of information at once while navigating an environment (a maze).1 Rats that received either 17b-estradiol (a naturally occurring estrogen) or a synthetic progesterone called levonorgesterol improved their working memory in a maze. However, rats that got both drugs together actually increased the number of errors made before solving the maze, suggesting that the drugs may act in opposition to each other rather than provide an added benefit.2
To explore how transitional menopause affects memory, Bimonte-Nelson and colleagues showed that VCD administration to young adult rats negatively impacted spatial working memory.3 They also demonstrated that administering conjugated equine estrogens (CEE; better known as Premarin), a common estrogenic hormone therapy, to middle-aged rats after VCD-induced transitional menopause impaired memory, but the same treatment enhanced memory after surgical menopause. Therefore, depending on the type of menopause model an animal experiences, hormone therapy can have divergent memory effects.4
Anxiety and Depression
Mary’s unusual sad moods and anxiety might be linked to her menopause transition.
Mood disorders are more prevalent in women than in men. Changes in reproductive hormone levels can have a profound impact on mood.
Serotonin is a neurotransmitter closely tied to mood regulation. The enzyme tryptophan hydroxylase-2 (TpH2) converts the amino acid tryptophan into serotonin. Estrogen increases TpH2 mRNA (helps make more serotonin in the brain). It may be no surprise that menopausal women (who have low estrogen) have decreased brain serotonergic activity and this may play a significant role in the onset of anxiety and depression during menopause. The Bimonte-Nelson lab found that surgically removing the ovaries in middle-aged rats decreased TpH2 mRNA, and administering CEE or 17b-estradiol increased TpH2 mRNA expression, which correlated with better spatial memory and less anxiety-like and depressive-like behaviors.5
This study corroborates the idea that estrogen is protective against depression and anxiety, and that estrogenic hormone therapy helps mitigate mood-related changes after surgical menopause.
Recovery After Stroke and Brain Injury
Mary has a family history of stroke, and wonders about how menopause and aging can influence her risk.
Estrogen plays a significant role in modulating normal cerebrovascular function to maintain homeostasis in several ways:
- Promotes anti-inflammatory molecules and regulates blood flow in the brain by acting at receptors located in specialized cells lining the brain’s blood vessels called endothelial cells.
- Mediates blood-brain barrier permeability (also via endothelial cells).
- Regulates mitochondria function and interacts with glia (the brain’s immune and support cells), to maintain a healthy brain.
Ovarian hormones are neuroprotective following injury. After menopause, women’s risk for stroke and cardiovascular disease increases. Estrogen can protect and heal the brain after damage like ischemic stroke or traumatic brain injury (e.g. preventing some post-injury brain cell death). Although clinical studies are not yet conclusive regarding estrogen-containing hormone therapy in stroke recovery, understanding how estrogen interacts with the brain circulation at a cellular and molecular level provides insight into future therapeutic options.6
Progesterone treatment has also been implicated in neuroprotection after brain injury. Animal models of stroke and traumatic brain injury indicate that giving a high dose of progesterone before or shortly after injury reduces the size of the damaged area, stimulates anti-inflammatory actions of glia, and enhances recovery of sensory-motor and cognitive function following the injury. These reproductive hormones can increase growth factors in the brain that put the recovery process in motion. Additionally, ovarian hormones are naturally metabolized to other biologically active substances. Allopregnanolone, a metabolite of progesterone, has been credited with neuroprotection after injury through its ability to modulate the inhibitory neurotransmitter GABA.7
Whether progesterone and estrogen work together or via different pathways to promote neuroprotection and enhance recovery prospects is still being investigated.
Neurodegeneration
Memory lapses are normal even in healthy aging, but menopause might influence Mary’s risk of cognitive decline and/or dementia
The World Health Organization estimates dementia currently affects about 47 million people worldwide, and by 2050 this number is projected to triple. Dementia is not a normal phenomenon of aging, but rather is an umbrella term for diseases and disorders that result in a progressive decline of memory, executive functioning, and the ability to independently complete everyday tasks. Alzheimer’s disease (AD) is one of these progressive neurodegenerative diseases which has some unique characteristics:
- AD has two specific neuropathology markers: amyloid-beta plaques and neurofibrillary tau tangles.
- AD presents in two main ways:
- Familial, early-onset (about age 40). Genetically heritable and comprises < 1% of total AD cases.
- Sporadic, late-onset (after age 65). People with a specific form of the apolipoprotein gene—ApoE-ε4—are at a higher risk
Two-thirds of diagnosed cases are women.
While neither sex differences in ApoE gene status nor increased female longevity fully account for the fact that women are disproportionately affected by AD, an interaction of these factors with the loss of estrogen and progesterone at menopause may help explain women’s increased susceptibility to AD with aging.8
Research from the Mayo Clinic showed oophorectomy before natural menopause and without subsequent hormone therapy increases the relative risk of developing AD. Interestingly, women who took estrogen-containing hormone therapy following oophorectomy until age 50 did not have an increased risk of developing AD, indicating some neuroprotective role for estrogen. Unfortunately, other large clinical trials have concluded that hormone therapy can actually increase dementia risk, which adds complexity to understanding ovarian hormones’ neuroprotective qualities.9
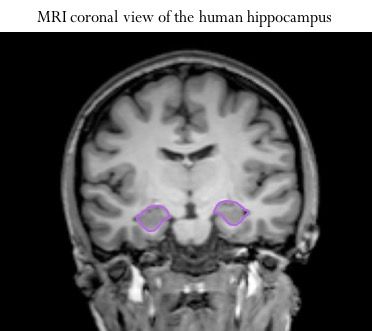
Back to the hippocampus: it is critical for learning and memory and loaded with estrogen and progesterone receptors. This brain structure is one of the most severely impacted by AD. Transgenic animal models of AD (human genes inserted in their DNA) suggest that ovariectomy can physically change the brain by exacerbating amyloid-beta plaque load in the hippocampus (similar to AD). Estrogen treatment can improve this.
Yet other laboratories report no change in AD-like pathology after ovary removal or estrogen treatment, and/or no cognitive enhancements following hormone administration. As such, estrogen loss and replacement does not yet have a proven causative impact on the development and progression of AD pathology, but clearly interacts with factors contributing to AD development, like inflammation and cerebrovascular changes, and thus is still a significant area of research interest.
The proposed “window of opportunity” for benefits of hormone therapy will likely also play a meaningful role in understanding the complex relationships among menopause, aging, the brain, and neurodegenerative disease.
Clearly, the effects of estrogen and progesterone go beyond the gonads.

Given that women are living longer lives, scientists such as Dr. Heather Bimonte-Nelson and colleagues at the Neuroscience of Memory and Aging Laboratory at Arizona State University strive to discover novel ways to promote healthy brain aging and maintain a high quality of life.
References
1. Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161-173.
2. Prakapenka AV, Hiroi R, Quihuis AM, Carson CC, Patel S, Berns-Leone C, Fox C, Sirianni RW, Bimonte-Nelson HA. Contrasting effects of individual versus combined estrogen and progestogen regimens as working memory load increases in middle-aged ovariectomized rats: one plus one does not equal two. Neurobiol Aging. 2018;64:1-14.
3. Koebele SV, Mennenga SE, Hiroi R, Quihuis AM, Hewitt LT, Poisson ML, George C, Mayer LP, Dyer CA, Aiken LS, Demers LM, Carson C, Bimonte-Nelson HA. Cognitive changes across the menopause transition: A longitudinal evaluation of the impact of age and ovarian status on spatial memory. Horm Behav. 2017;87:96-114.
4. Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151(8):3795-3804.
5. Hiroi R, Weyrich G, Koebele SV, Mennenga SE, Talboom JS, Hewitt LT, Lavery CN, Mendoza P, Jordan A, Bimonte-Nelson HA. Benefits of hormone therapy estrogens depend on estrogen type: 17beta-estradiol and conjugated equine estrogens have differential effects on cognitive, anxiety-like, and depressive-like behaviors and increase tryptophan hydroxylase-2 mRNA levels in dorsal. Front Neurosci. 2016;10(DEC):1-20.
6. Engler-Chiurazzi EB, Brown CM, Povroznik JM, Simpkins JW. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog Neurobiol. 2016.
7. Singh M, Su C. Progesterone-induced neuroprotection: Factors that may predict therapeutic efficacy. Brain Res. 2013;1514:98-106.
8. Riedel BC, Thompson PM, Brinton RD. Age, APOE and Sex: Triad of Risk of Alzheimer’s Disease. J Steroid Biochem Mol Biol. 2016;160:134-147.
9. Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Res. 2011;1379:188-198.




