Addiction
Understanding Addiction With Electronic Registries
Disruptive technology with a $40 per patient clinical trial price tag.
Posted November 18, 2015
The randomized clinical trial (RCT) is the gold standard for clinical research. In an RCT, patients are recruited and enrolled into a study, assigned one treatment or another randomly, and followed-up as their disease of interest progressed or improved. Outcomes are compared between the treatment groups, and standard statistical tests are used to analyze the results to ascertain whether one treatment is better than the other.
RCTs are the basis of establishing effectiveness for the vast majority of medical treatments, and are used for Food and Drug Administration (FDA) and other governmental agencies in the approval for specific medications or medical devices. RCTs and related methodologies have also deeply contributed to a wide variety of other disciplines, including public policy, marketing and operations research, and education.
However, RCTs have a few problems:
First, RCTs are expensive. It costs about $15,000 conservatively to randomize one patient in a traditional RCT. Effect sizes are often small, and many patients have to be enrolled. For a modestly sized study of 100 patients, simply getting enough patients would cost $1.5 million. This is not counting the cost of the expertise for analysis, execution, and safety monitoring throughout the study.
Second, RCTs may not be generalizable. You can test treatments and follow patients in ideal situations, but people in the real world do unpredictable things. They drop out of treatment. They move. They develop other diseases that would disqualify them from entering the study in the first place. Are the results from your study really applicable in the real world?
Here is the Uber-ification idea for RCTs: is it possible to peg electronic medical records to a centralized research facility so that each time a diagnosis is made in the community it automatically triggers a protocol that begins the enrollment, consent, randomization, follow-up process? Safety monitoring would be conducted by the physicians the patients see, and improving generalizability would be a no-brainer.
This is the idea behind this study: Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) trial. The New England Journal of Medicine hailed it as the next "disruptive" technology in clinical trials. The perspectives article, which aims at non-specialists, is worth a read. In the TASTE study, people with a specific kind of heart attack were randomized to either getting thrombus aspiration or not (which is a procedure that occurs during "Percutaneous Coronary Intervention", a jargon that means, colloquially, stenting). The most remarkable thing is that they were able to randomize 5000 patients at around $40 each, by using a standardized electronic medical records system.
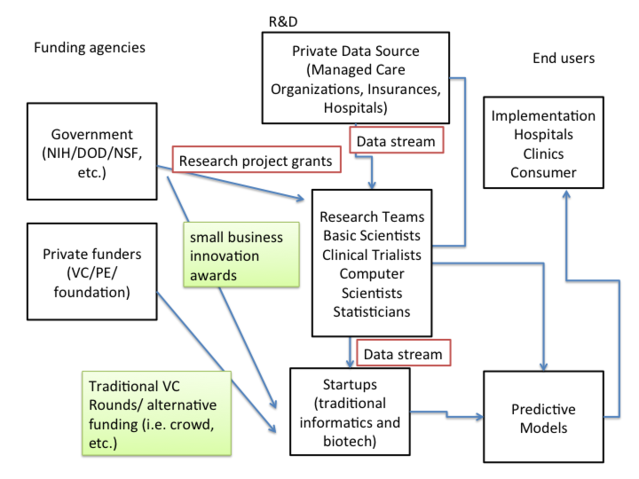
Maybe this is only doable in Scandinavia. Maybe not. We probably have the resources to do this here in the U.S., if we can leverage productive private-public collaborations. The enrollment of large integrated care systems (e.g. Kaiser) is larger than the entire populations of some Scandinavian countries. And while it is often hard to make regulatory headway (i.e. centralizing EMR) in the US, it tends to be faster to push these initiatives in the US through in a private system with large Managed Care Organizations/Venture Capital/foundations and other alternative funding sources.
So what are some questions in addiction I would like answered if we had a system like that? I would perhaps consider re-doing PROJECT MATCH for psychotherapy. I would consider a large RCT on the efficacy of telemedicine-driven TES. Of course various medication comparisons. What else should we consider? What is an easy outcome? For heart attacks mortality is the easiest outcome. For substance abuse, it's not so easy. The leading organization in large multi-center RCTs substance abuse research is the National Institute of Drug Abuse Clinical Trials Network and people just started thinking about these issues in the newly formed Data Science Task Force, and certainly all of us are dying for all the help and ideas we can get.
This blog is a condensed version of a longer column at Rehabs.com.


