Ketamine
Why Is Ketamine Still Considered Investigative?
An unclear insurance label may impact those with treatment-resistant depression.
Posted April 12, 2023 Reviewed by Kaja Perina
Key points
- Insurance coverage for ketamine is spotty at best, with insurers often giving the drug an inconsistently defined ‘investigative’ label.
- This status severely limits access for many patients whose lives could be greatly improved by ketamine.
- Ketamine should not be considered investigative, should be covered by insurance, and encouraged for severely depressed and suicidal patients.

This post was co-authored by Stephen Manlove
Ketamine has become one of the most effective and frequently used drugs for debilitating mental health conditions such as treatment-resistant depression, anxiety and PTSD. Yet insurance coverage for ketamine is spotty at best, with insurers often giving the drug an inconsistently defined ‘investigative’ label as reason for denying coverage.
This ‘investigative’ status severely limits access for many patients whose lives could be greatly improved by ketamine. Meanwhile, experienced mental health providers have confidence in the safety of ketamine and are convinced that the drug is an excellent psychopharmacological addition when administered in safe conditions by mental health professionals.
Ketamine is known to reduce suicidality1. It is common knowledge that we are in a mental health epidemic with depression and suicidality leading the way. When our country was faced with the COVID pandemic our country was able to ramp up very quickly and develop a vaccine which likely saved hundreds of thousands of lives. The federal government implemented six different pieces of legislation to provide support to individuals, business, and state and local governments. More than $30 billion was spent on COVID vaccines to encourage their development, guarantee a market, and ensure that the public could access them at no charge.
It is now time to focus on the mental health epidemic. We already know that ketamine should be a significant part of this project.
Ketamine should no longer be considered investigative, should be covered by insurance, and encouraged for severely depressed and suicidal patients for the following reasons:
- Ketamine is in general use in the medical community: Minnesota, for example, has at least thirteen clinics that use ketamine to treat depression, including the Mayo Clinic and the University of Minnesota, plus several more which utilize esketamine (the FDA-approved nasal spray form which has the same active ingredient as ketamine). Nationwide, hundreds of medical clinics use ketamine to treat depression, including some publicly traded companies. Internationally, ketamine is on the World Health Organization’s list of essential medications.
- Ketamine has been proven safe and effective—in fact it can be life-saving—and shows a demonstrable benefit for depression and anxiety patients who have often found no relief from traditional antidepressants. There is ample available scientific evidence (see References) that leads our team to conclude that ketamine for depression has a positive effect on health outcomes with few side effects if properly used.
- Ketamine is undergoing ongoing scientific testing and research. Ketamine-related research is increasing, with recent FDA approval of clinical trials for R-ketamine.
- The consensus statement of a pivotal 2021 paper2—authored by some of the world’s top psychopharmacologists—summarizes the position of the American Psychiatric Association and cites pertinent literature supporting the position. The study’s conclusion, upon reviewing the risks and benefits of esketamine and ketamine states, “In the interim, these agents should be administered only at centers with the appropriate infrastructure and multidisciplinary personnel with expertise in the assessment and treatment of adults with mood disorders.”
- A 2021 study comparing the efficacy of racemic ketamine and esketamine for depression3 supports the superiority of ketamine over esketamine. It documents that ketamine has approximately twice the response and remission rate as esketamine and has a much lower dropout rate. The study was published in a well-respected medical journal with an impact factor of 4.8, and the authors are top-rated international researchers.
4. Ketamine is needed for treatment-resistant depression. In 2004, the Star D study4 found thirty percent of depressed patients do not respond to traditional antidepressants. The more antidepressants a patient has failed, the less likely it is that the next antidepressant will be effective. Treatment-resistant depression is defined as when a patient fails two or more antidepressants. Ketamine works differently than standard antidepressants and may be helpful for patients who need a different mechanism of action. A 2021 study5 shows ketamine is about as effective as the gold standard for treatment-resistant depression, electroconvulsive therapy (ECT), without the concomitant morbidity of ECT.
5. Ketamine can be safely used in a structured outpatient setting. For patients with treatment-resistant depression and suicidality, ketamine is the lowest level of care that may effectively treat their problem. ECT requires a higher level of care. Transcranial Magnetic Stimulation requires a slightly lower level of care but also requires special equipment and is not effective for all patients with treatment-resistant depression.
6. There is a safe treatment protocol. When the FDA approved esketamine for the treatment of depression in 2019, a REMS program was developed to allow safe administration and monitoring of esketamine.
7. Ketamine is economically efficient in its use of medical services and supplies that may be provided safely and effectively to the patient, compared to using the single branded version of esketamine. See Table 1 below for a drug and treatment cost comparison.
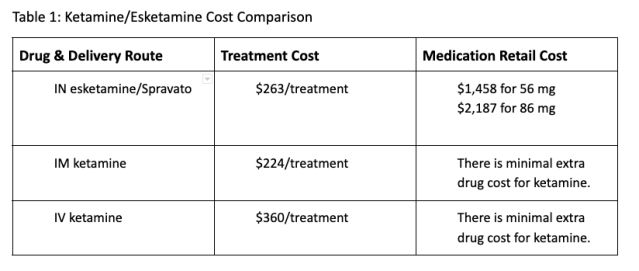
When you factor in that intramuscular and intravenous delivery of ketamine is considerably more effective than intranasal delivery, the ethical and economic choice in favor of ketamine is clear for physicians.
Conclusion
Considering the above information and the References listed below, a consensus has recently emerged among qualified medical professionals: ketamine for treatment-resistant depression should no longer be considered investigative. Given the debilitating and life-threatening nature of treatment-resistant depression, anxiety and PTSD, the mental health community and our patients need ketamine–in all its forms–as a treatment option. And in too many cases, insurance coverage is the only barrier. Roadblocks should be removed which prevent patient access to ketamine.
References
1 Mocrane Abbar, chief of clinical department, Christophe Demattei, statistician, Wissam El-Hage, chief of clinical department, Pierre-Michel Llorca, chief of clinical department, Ludovic Samalin, assistant professor of psychiatry, Pierre Demaricourt, chief of clinical department, Raphael Gaillard, chief of clinical department, Philippe Courtet, chief of clinical department, Guillaume Vaiva, professor of psychiatry, Philip Gorwood, chief of clinical department, Pascale Fabbro, methodologist, Fabrice Jollant, professor of psychiatry (2022). Ketamine for the acute treatment of severe suicidal ideation: double blind, randomised placebo controlled trial. BMJ 2022; 376 doi: https://doi.org/10.1136/bmj-2021-067194 (Published 02 February 2022).
2 Mcintyre, R. S., Rosenblat, J. D., Nemeroff, C. B., Sanacora, G., Murrough, J. W., Berk, M., Brietzke, E., Dodd, S., Gorwood, P., Ho, R., Iosifescu, D. V., Lopez Jaramillo, C., Kasper, S., Kratiuk, K., Lee, J. G., Lee, Y., Lui, L. M.w., Mansur, R. B., Papakostas, G. I., . . . Stahl, S. (2021). Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: An international expert opinion on the available evidence and implementation. American Journal of Psychiatry, 178(5), 383-399. https://doi.org/10.1176/appi.ajp.2020.20081251
3 Bahji, A., Vazquez, G. H., & Zarate, C. A. (2021). Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. Journal of Affective Disorders, 278, 542-555. https://doi.org/10.1016/j.jad.2020.09.071
4 Rush A, Fava M, Wisniewski S, et al. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Control Clin Trials 2004; 25:119–142
5 Ekstrand, J., Fattah, C., Persson, M., Cheng, T., Nordanskog, P., Åkeson, J., Tingström, A., Lindström, M. B., Nordenskjöld, A., & Movahed rad, P. (2021). Racemic ketamine as an alternative to electroconvulsive therapy for unipolar depression: A randomized, open-label, non-inferiority trial (KetECT). International Journal of Neuropsychopharmacology, 25(5), 339-349. https://doi.org/10.1093/ijnp/pyab088




