Dementia
Glial Cells and Plaque Buildup in the Alzheimer's Brain
Potential for new Alzheimer's therapies.
Posted August 26, 2024 Reviewed by Monica Vilhauer
Key points
- New research indicates a possible source for the misshaped proteins indicative of AD.
- Age-related degeneration of a glial cell called an oligodendrocyte may trigger plaque formation.
- Research aims to identify where and why waste products accumulate to design a treatment to reverse it.
The human brain is a three-pound wonder, capable of amazing things right alongside everyday behaviors so automatic we don’t even notice they’re there. The brain is made up of two kinds of cells. Neurons, which send messages and commands to the rest of the cells of the brain and body, letting us act, think, and plan, and glial cells which maintain our neurons and clean up after them.
For a long time, neurons were considered to be the most important cells in the brain because of their message-sending and receiving capabilities. However, recent evidence suggests that glial cells are more than just housekeepers for the CNS. They also appear to have a role in synaptic plasticity (change in the ways neurons connect to one another, shaped by experience), learning, and memory.
Most of the time the glial and neuronal cells operate outside of our awareness. For example, I am not aware of the individual messages the cells in my brain are sending as I write this, and clean-up by the glial cells typically happens when we’re sleeping. But every once in a while, something goes wrong with one of the systems and our behavior suffers as a result. If you’ve had a loved one suffer from a neurodegenerative disease, you’ve unfortunately seen this up-close and personal.
Researcher Maiken Nedergaard sums up neurodegenerative diseases quite simply. He says that “essentially all neurodegenerative diseases are associated with mis-accumulation of cellular waste products” (Nedergaard, 2013, page 1529).
Plaques and Alzheimer’s Disease

In what is probably the best-known neurodegenerative disease, Alzheimer’s Disease (AD), these waste products take the form of Beta-Amyloid proteins which form plaques. Plaques are clumps of pieces of these proteins that accumulate outside of the neurons of the brain. They surround the neurons and can block the messages that cells normally send to one another, trigger inflammation and ultimately have a toxic effect on brain cells. Beta-Amyloid proteins interact with another kind of protein called tau proteins that make up the skeletal structure of the neurons, causing them to clump together inside the cells forming what are called tangles. These tangles inside the neurons cause the internal structure of the neuron to fall apart and disrupt the functioning of the nerve cells. The presence of plaques outside of the neurons in the brain along with the presence of tangles inside the cells are the hallmarks of AD.
Proteins are constructed of amino acids which are then folded into their appropriate shape. Sometimes the folding process goes awry. The misshapen proteins are either nudged back into their proper formation or they are gotten rid of via a system similar to the lymph system that clears the rest of your body of infection and old or damaged cells it does not need. Nedergaard coined the term glymphatic system for the neural version of the lymph system, for its similarity to the peripheral lymph system, and for the brain cells that are in charge of waste removal, the glial cells. A failure of this clean-up process can result in the accumulation of these malformed proteins into plaques. This makes understanding the process of metabolizing these proteins important in developing treatments for AD and other neurodegenerative disorders.
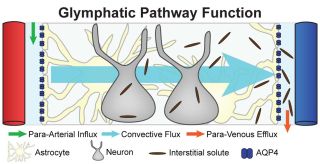
Oligodendrocytes and Clean-Up
Originally, researchers thought that beta amyloid proteins were being generated by excitatory neurons in the brain, but more recent evidence suggests that both neurons and glial cells are contributing to the beta amyloid burden in the CNS. Researchers (Sasmita, Depp, Nazarenko, et al., 2024) found that a type of glial cell called oligodendrocytes is particularly involved. Oligodendrocytes serve an important function in the brain. They create myelin sheaths around the axons of neurons, allowing for faster and more efficient message sending by cells. Loss of myelin is another symptom of AD and a factor in the disruption of brain function in the disorder. Depp et al. (2023) reported that abnormal myelin function, associated with aging, drives Beta-Amyloid deposition.
Specifically, studies have shown that myelin plays an important role in learning and memory, synchronizing the input to cells in the hippocampus, the portion of the brain primarily responsible for the creation of memory. Oligodendrocytes accomplish this by facilitating the speed and timing of the arrival of messages to the hippocampus, and by affecting the way that input is integrated by hippocampal cells. Disruption of the myelin sheath creates disruption of memory, the main symptom of AD. (Munyeshyaka and Fields, 2022). However, precisely why myelin function is disrupted with aging is still unknown.

One focus of the research currently being done on neurodegenerative diseases is figuring out where these waste products are coming from and why they are accumulating. The thinking is that if we can identify where and why the accumulation of Beta-Amyloids begins, we can design a treatment to reverse or slow this accumulation and in doing so, reverse or slow AD.
References
Depp, C., Sun, T., Sasmita, A.O. et al. (2023). Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature, 618, 349–357, doi.org/10.1038/s41586-023-06120-6.
Munyeshyaka M, Fields R.D., (2022). Oligodendroglia are emerging players in several forms of learning and memory. Communications Biology, 5(1), 1148, doi.org/10.1038/s42003-022-04116-y.
Nedergaard, M., (2013). Garbage truck of the brain, Science, 340(6140), 1529-1530. doi:10.1126/science.1240514


