Genetics
Babies With 3 Biological Parents
Some techniques for assisted reproduction introduce DNA from a third individual.
Posted December 4, 2020
Mitochondria and mitochondrial DNA (mtDNA)
Mitochondria are organelles that generate the energy powering our cells, and carry small circular chromosomes. Mitochondrial DNA (mtDNA) is distinct from the bulk of our DNA, which is found in the cell nucleus. Although only a tiny proportion of the total human genome, mtDNA is critically important to energy production in the mitochondria. Although there are only a few genes present in mtDNA, there are generally many copies per cell, as every cell has multiple mitochondria each containing several copies of the circular chromosome.
Mutations occur in mtDNA, and can lead to debilitating and potentially fatal genetic diseases. All mitochondria, and mtDNA, come from the mother. Human sperm only contribute nuclear DNA during fertilization, while each egg contains a large heterogeneous mix of mitochondria with different numbers of copies of the mtDNA chromosome, with specific genetic variants, including potential mutations. In this way, a woman who is a carrier for a genetic disease caused by a mutation in mtDNA can have some children who are unaffected, and others who have the disease. As this does not follow conventional patterns of "Mendelian" inheritance, which requires one maternal and one paternal copy of each gene as found in nuclear DNA, predicting the outcomes of these situations can be extremely complex. So both genetically and medically, understanding, preventing, and treating diseases that originate from mutations in mtDNA is a major challenge.
Mitochondrial replacement therapy (MRT)
Mutations in mtDNA can cause debilitating human diseases, including a number of rare neurological disorders such as Kearns-Sayre syndrome, Leber’s hereditary optic neuropathy, and Leigh syndrome. Thus, innovative techniques have been developed to assist would-be mothers with a significant risk of having children affected by mitochondrial dysfunction. Many of these interventions involve in vitro fertilization (IVF), the combination of sperm and egg in the laboratory before implantation into the prospective mother. If IVF is performed with donor eggs, the baby will carry on the genetics of the donor. However, rather than using donor eggs, these procedures can include injecting the ooplasm (the material inside of the egg, which includes mitochondria, but excludes the nucleus) donated by a woman with no risk of carrying mutations in mtDNA, into an oocyte collected from the affected would-be mother before IVF.
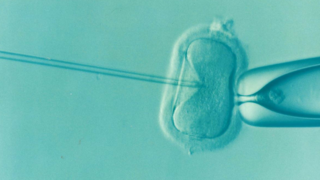
Ooplasm injection allows for healthy mitochondria without mtDNA mutations to be introduced into the fertilization process. Other techniques have been developed to provide mutation free mtDNA to a child conceived by IVF, but what each has in common is that mitochondria and mtDNA in the resultant child come from the donor, but the nuclear genome comes from the parents. Thus, biologically the baby has three parents.
These techniques, which have been possible for many years, are collectively referred to as mitochondrial replacement therapy (MRT). In the United States, the FDA strictly prohibits MRT. This national moratorium includes experimental application in any MRT research that might result in a live birth of a human child with three parents as well as any clinical application. It is currently unclear exactly what the legal, ethical, and biological implications might be of having a child with three parents, two who contributed nuclear DNA and one that provided mtDNA. However, this has not stopped some doctors in other countries from helping would-be parents through MRT techniques.
Beyond cases where the goal is to avoid mitochondrial disease, these procedures have also been shown to be potentially helpful as an infertility treatment. This is particularly relevant to cases where the inability for a couple to conceive seems to stem from issues with the oocytes. Advanced maternal age can be associated with the presence of nonviable eggs linked to issues with maternal mitochondrial function. Ranging from experimental studies to clinical application to avoid mitochondrial disease, as well as assisted reproduction in general, this is a complex area with many different points of view.
When IVF fails because of nonviable eggs, donor eggs are the only alternative currently available. However, this means that the nuclear and mtDNA in the offspring will not be that of the mother. MRT produces children where the genome is a combination of the mother and father, apart from the relatively small amount of mtDNA, which comes from the donor. MRT to avoid transmitting mitochondrial disease is legal in the UK, and there are certain doctors worldwide who are employing MRT to assist couples to conceive who have failed multiple rounds of IVF because of issues with oocyte viability.
What will the future hold?
Beyond using donor eggs for IVF, a surrogate, or adoption, there are few options for women with a high risk of having children with mitochondrial disease or who cannot generate viable eggs. For a woman with mutations in some copies of the mitochondrial genome, it can be extremely difficult to know the potential outcome of any specific pregnancy. While it is possible to genetically screen early embryos for the presence of mutations in mtDNA, given the complex nature of mitochondrial inheritance, there is no fixed threshold that can predict absolutely whether a child at risk will in fact suffer from a mitochondrial disease. Furthermore, female children born after this screening would still carry the risk of transmitting mitochondrial disease to their children. Interestingly, given the metabolic requirements of early embryonic development, it may well be that some effects of mutations in mtDNA might actually prevent pregnancy from progressing. Although this doesn’t necessarily help with the problem of improving reproductive success in women who carry these mutations, it might ultimately reduce the risk of giving birth to children affected with mitochondrial disease.

Although many women who carry potentially disease-causing mutations in mtDNA, and those who otherwise cannot generate viable eggs, want to have children—and a potentially effective option seems to exist—legal, ethical, and regulatory boundaries are preventing many couples from becoming biological parents. The concerns that have led to these regulations are certainly valid. However, as we have seen with the potential application of CRISPR to human genome editing, creating rational and reasonable international guidelines and regulations for applying genomic technologies to modifying the human species is not simple in any way. The question of what the future will bring is a big one. As individuals and as a species, we seem to be hovering on the brink of unlocking myriad novel ways in which genomic technologies may revolutionize many facets of life as we know it.


