Pregnancy
The Sperm that Fertilizes: Champion Swimmer or Lucky Winner?
Images of racing sperms are overblown — passive transport is also crucial
Posted March 28, 2019
Picture a horde of sperms frantically racing towards the egg. The recent British documentary The Great Sperm Race dramatically underscores this notion, which is deeply ingrained in the public imagination as well as in scientific reports. Yet, as my 2018 essay, The Macho Sperm Myth in Aeon magazine shows, the idea of racing sperms is deceptive. People often assume that the sperm that fertilizes the egg is some kind of champion, like a victorious swimming competitor. But why assume that the most rapid swimmer must be best for fertilization? While the 250 million sperms in the average human ejaculate admittedly include many duds, there is no convincing evidence — for healthy, active sperms of normal shape — that success in fertilization has any connection with overall genetic quality.
Long-neglected evidence that sperms are stored in the human womb (see What Dogs Can Tell Us About Sex and Conception, posted on March 3, 2016) strikingly illustrates the shortcomings of sperm race thinking. When sperms pass up the neck of the womb (cervix), some migrate directly into the womb cavity, but others wander into blind side-chambers (crypts), where they can remain for over a week. Several authors see such sperms as losers that strayed in the wrong direction and missed their chance to win the prize of fertilization. However, it is highly likely that sperms in crypts are protected and held in reserve for gradual release. Regrettably, because of researchers’ preoccupation with sperms racing towards the egg, only one paper — published by Vaclav Insler and others in 1980 — has seriously examined sperms stored in the crypts of the human womb.
Hitching a ride
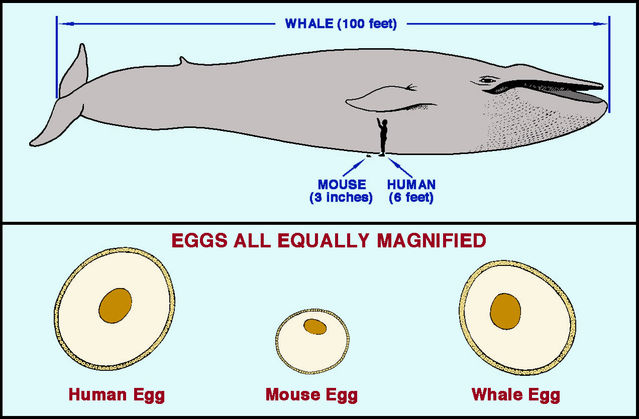
In fact, without direct examination, we can easily ditch the idea that sperms race all the way to the egg in mammals. Consider this: Do bigger bodies have more cells, larger cells or something in between? Surprisingly, cell size is remarkably uniform across mammals; bigger mammals simply have more cells. As Carl Hartman noted in 1962, this also applies to eggs and sperms, which have approximately similar sizes in small mammals such as mice, medium-sized mammals like humans, and blue whales (the largest mammals alive today). Sperms might just be able to swim all the way up the female tract in mice, but this is physically impossible for blue whales, which weigh around 10 million times more. To reach the egg in a blue whale, a sperm must travel some 400 times farther than a mouse sperm of similar size! For large mammals at least, sperms clearly need assistance to reach the egg.
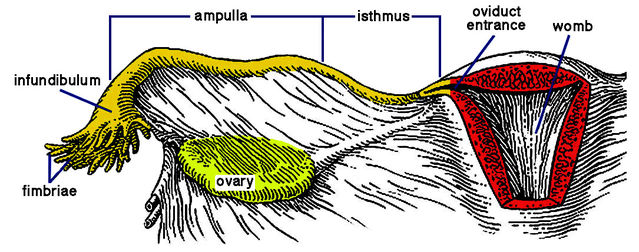
As medium-sized mammals, women likewise need mechanisms to assist sperm transit. To reach the egg after semen deposition deep in the vagina, sperms must travel more than eight inches — through the cervix, across the womb cavity, and up an oviduct to reach the fertilization site at its upper end (ampulla). Top swimming speed of unaided human sperms is about seven inches per hour. So, even assuming it carries enough “fuel” for such a feat, a sperm would need over an hour to swim to the egg. Yet many studies of women and other mammals have revealed that some sperms arrive in the ampulla within minutes of insemination.
Contraction waves in the womb and oviducts
In fact, involuntary muscular contractions in a woman’s womb have been known since 1889. Various experiments have clearly revealed wave-like contractions, resembling the peristalsis that propels food from the mouth down the food pipe (oesophagus) to the stomach. In 1991, Edward Lyons and colleagues reported results of ultrasound scanning using a vaginal probe to record contractions in the wombs of 18 women volunteers. This study was particularly informative because it examined changes across the menstrual cycle. Around mid-cycle, most contraction waves passed from the cervix to the top of the womb, but the main direction was reversed before and after menstruation. This indicates that close to ovulation the womb pumps sperms into the oviducts and towards the ovaries.
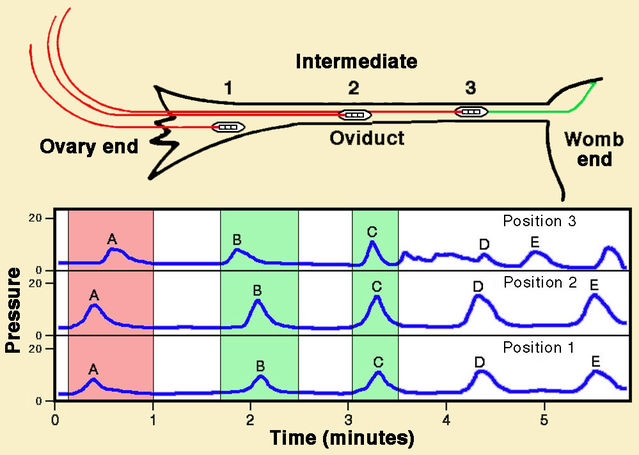
Far less information is available regarding a pumping action of the oviducts. However, in 1970 Hugo Maia and Elsimar Coutinho reported direct recordings of muscular activity along the oviduct in seven patients, using pressure-sensitive probes. Intriguingly, here too contractions occurred in both directions, passing mainly down the oviduct towards the womb but sometimes in the opposite direction. Contractions passing up the oviduct were more frequent at mid-cycle.
Migration of inert particles
Some investigators used inert particles, rather than actual sperms, to study transport mechanisms. Early findings from cows indicated that some sperms enter the oviduct less than three minutes after insemination, and comparable reports emerged from human medicine. In 1972, Charles de Boer published findings from a large sample of almost 200 patients scheduled for either hysterectomy or oviduct tying. Before surgery, he injected small quantities of India ink (carbon particles suspended in water) at various sites in the vagina or womb. Ink migrated from the vagina to the oviduct in only 6% of patients. By contrast, transfer from the cervix to oviduct ensued in almost a third, while transit from the womb chamber occurred in over half. In some patients, substantial amounts of ink spilled into the abdominal cavity after passing along an oviduct.
X-ray images of pumping wombs and oviducts
In 1991, Thomas Steck and colleagues reported results from a novel approach to studying sperm transport. They combined deposition of inert particles with hysterosalpingography (HSSG), a procedure that uses X-ray imaging to examine the womb and oviducts. Sperm-sized albumin spheres labeled with technetium (a radioactive tracer with a very short half-life) were deposited deep in the vagina near the cervix, and their migration was subsequently followed with a detector. Spheres entered oviducts rapidly, as early as one minute after deposition at the entrance of the cervix, clearly revealing a pump-like action of the womb.
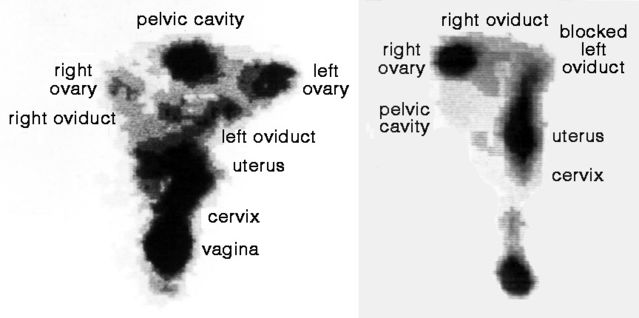
HSSG subsequently became a standard technique in fertility clinics for direct study of passive transport of albumin spheres through the womb and oviducts, as notably described in a series of papers by Georg Kunz, Ludwig Wildt, and others. In 1996, they reported findings from 64 women using HSSG. Contraction waves ensured rapid and sustained transport from the cervix to the oviducts, especially near ovulation time.
HSSG also yielded a striking finding regarding the trajectory of albumin spheres through the female reproductive tract. A woman typically releases only one egg per cycle, from either the left or right ovary. To fertilize the egg efficiently, a sperm must travel up the appropriate oviduct. Kunz and colleagues confirmed that spheres were mainly carried along the oviduct on the side of egg release.
But sperms do need to swim
Sperms evidently benefit from passive transport, especially in large-bodied mammals. Pumping actions of the womb propel them into the oviducts and contractions of the oviducts then convey them towards the fertilization site. It is essentially up to chance which one ends up fertilizing the egg. Nonetheless, normal sperms most definitely do have impressive swimming capacities. These are particularly important during passage from the vagina into the cervix, from the womb into an oviduct, and in the final lead-up to fertilization of the egg.
Remarkably, an unusual method of artificial insemination involves injection of sperms into the abdominal cavity rather than the womb, exploiting their persistent swimming capacities. Yves Rumpler and colleagues first reported this method of direct intraperitoneal insemination (DIPI) in 1986. A decade later, Caroline Tiemessen and others reported that DIPI was surprisingly successful compared with routine insemination into the womb (16% pregnancy rate per cycle versus 24%). It is amazing that fertilization occurred at all!
An even more unusual finding confirms that sperms can swim very effectively if necessary. Some women have a blocked oviduct, often following infection. If the blockage is on the same side as the ovary releasing the egg, fertilization is unlikely. But several reports show that fertilization can nevertheless occur in such cases. The only explanation is that the fertilizing sperm migrates up the unblocked oviduct and then swims across the abdominal cavity to reach the egg released from the ovary on the opposite side. The embryo implants and develops in the upper end of the blocked oviduct, so surgery is unfortunately obligatory. But this does bear eloquent testimony to the dogged swimming capacities of sperms heading towards the egg.
References
Ansari, A.H. & Miller, E.S. (1994) Sperm transmigration as a cause of ectopic pregnancy. Archives of Andrology 32:1-4.
Brannigan, R.E. & Lipshultz, L.I. (2008) Sperm transport and capacitation. Global Library of Women's Medicine 1-13. DOI:10.3843/GLOWM.10316
Channel 4 TV & Wellcome Trust (2009) The Great Sperm Race (Human Anatomy Documentary). Narrated by Richard Armitage.
https://trakt.tv/shows/channel-4-uk-documentaries/seasons/2009/episodes…
de Boer, C.H. (1972) Transport of particulate matter through the female genital tract. Journal of Reproduction & Fertility 28:295-297.
Forrler, A., Dellenbach, P., Nisand, I., Moreau, L., Cranz, C., Clavert, A. & Rumpler, Y. (1986) Direct intraperitoneal insemination in unexplained and cervical infertility. Lancet 327:916-917.
Insler, V., Glezerman, M., Zeidel, L., Bernstein, D. & Misgav, N. (1980) Sperm storage in the human cervix: a quantitative study. Fertility & Sterility 33:288-293.
Hartman, C.G. (1962) Science and the Safe Period: A Compendium of Human Reproduction. Baltimore: Williams & Wilkins Co.
Kunz, G., Beil, D., Deininger, H., Wildt, L. & Leyendecker, G. (1996) The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Human Reproduction 11:627-632.
Maia, H.S. & Coutinho, E.M. (1970) Peristalsis and antiperistalsis of the human Fallopian tube during the menstrual cycle. Biology of Reproduction 2:305-314.
Steck, T., Wurfel, W., Becker, W. & Albert, P.J. (1991) Serial scintigraphic imaging for visualization of passive transport processes in the human Fallopian tube. Human Reproduction 6:1186-1191.
Suarez, S.S. & Pacey, A.A. (2006) Sperm transport in the female reproductive tract. Human Reproduction Update 12:23-37.
Tiemessen, C.H.J., Bots, R.S., Peeters, M.F. & Evers, J.L. (1997) Direct intraperitoneal insemination compared to intrauterine insemination in superovulated cycles: a randomized cross-over study. Gynecologic & Obstetric Investigation 44:149-152.




