Genetics
Mitochondria: Missing Pieces in Imprinted Brain Epigenetics?
DNA inherited only from the mother may play a key role in brain development.
Posted January 30, 2021 Reviewed by Gary Drevitch
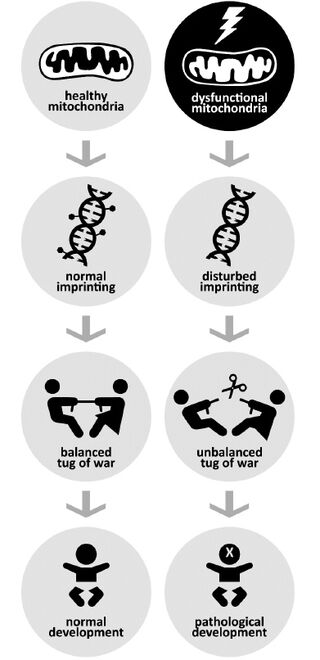
From the beginning, it was likely that mitochondrial genes, which are inherited only from the mother, ought to play a role in the imprinted brain theory, simply because it claimed that brain development was the result of a conflict between genes from the parents. But what role? And how could just 37 mitochondrial genes outside the nucleus and far away from the central scene of the genetic action be important? In an earlier post, I gave the best answers I could at the time, but now a paper by Paola Bressan and Peter Kramer (illustrated at left) puts the missing piece of the puzzle into place:
Since deleterious mutations that harm only males cannot possibly be purged by selection, then, mitochondrial genes have evolved to benefit women more than men [...]. Tellingly, mitochondrial DNA variants that reduce male fertility are quite common [...]. It has indeed been speculated that mitochondria—which have no evolutionary interest whatever in being in a male—could be selected to kill or feminize males during intrauterine development [...]. Yet this propension would seem only fair, considering the equally provocative proposal that some regions of the Y chromosome may push toward faster implantation and development of male embryos, leading to preferential abortion of females [...]; ... The preconditions for a battle of the sexes between mitochondrial and nuclear genes appear to be in place, as the two sets can influence one another in many different ways [...].
As these authors point out, the symptoms of epigenetic disorders (that is, those affecting gene expression) turn out to be strikingly similar to those of mitochondrial disease. Methylation is a key factor, and the expression of genes that encode it is upset by a common mutation in mitochondrial DNA. The authors note that removing the mitochondrial DNA from a cell massively changes the methylation of the cell’s genes, and that these aberrations can be partly restored by re-introducing it. They go on to point out that an important gene implicated in silencing by methylation, MECP2, is on the X chromosome, which is duplicated in mammalian females, but exists in a single copy in males:
Mutations of this gene are associated with Rett syndrome and PPM-X syndrome, two disorders that are roughly one another’s mirror image. Rett syndrome is due to a defective MECP2 gene on the paternally inherited X chromosome [...] and affects almost exclusively women, as only daughters normally receive this chromosome. The disorder comes with autism-like symptoms [...]. PPM-X syndrome is due to a defective MECP2 gene on the maternally inherited X chromosome; because, in sons, a faulty maternal X chromosome cannot be compensated by a paternal one, men feature the full disorder, whereas women may show no symptoms or a mild intellectual disability only [...]. PPM-X syndrome is strongly associated with manic–depressive psychosis [...]. Interestingly, MECP2 duplication syndrome in boys, in which the MECP2 gene is not defective but duplicated, is accompanied by autistic symptoms instead [...].
... just as the imprinted brain theory would expect and as the authors' table below illustrates:
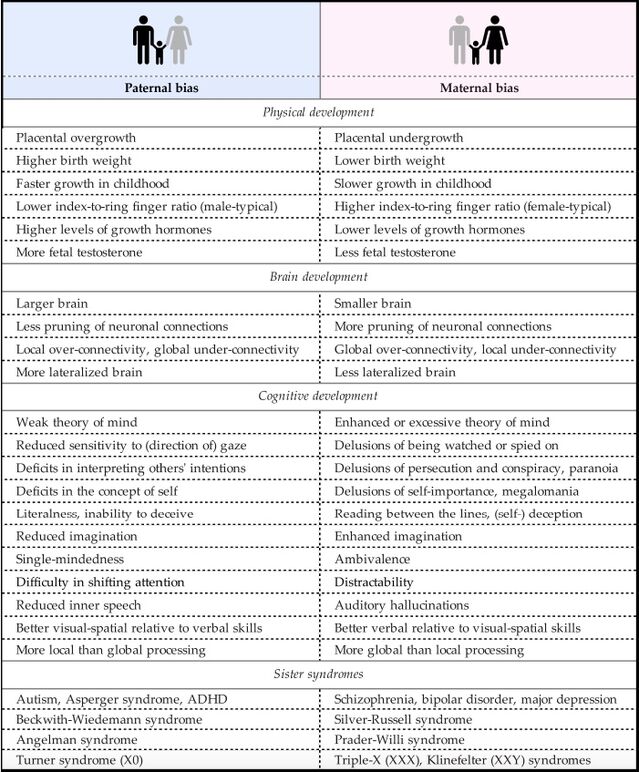
Bressan and Kramer add that Rett syndrome has many characteristics in common with mitochondrial disorders, ranging from delayed development and muscle weakness to seizures and heart problems, and that there is evidence that, directly or indirectly, the MECP2 gene affects the expression of nuclear genes that regulate mitochondrial functions. In fact,
... cells of Rett-syndrome patients, just like healthy rodent cells from which the MECP2 gene has been knocked out, contain malformed, poorly functioning mitochondria that produce little energy and a lot of free radicals. Anti-inflammatory dietary supplements that neutralize free radicals, such as fish oil, improve Rett symptoms in girls, suggesting that mitochondrial dysfunction contributes to the syndrome rather than being a mere side effect of it [...]. Furthermore, some patients who, based on their behavioral symptoms, are classified as classic Rett-syndrome patients do not carry a defective MECP2 gene at all but harbor mutations in their mitochondrial DNA [...].
Indeed, and as the table above also illustrates:
Dysfunctional mitochondria and high levels of free radicals have also been linked to Beckwith–Wiedemann [...], Angelman [...], and Prader–Willi [...] syndromes, as well as autism [...] and schizophrenia [...]. In fact, they have been observed in virtually all mental afflictions [...], from chronic psychological stress [...] and fatigue [...] to Alzheimer’s and Parkinson’s disease [...].
As these authors also comment, mitochondrial disease can show up with primarily psychiatric symptoms, and mutations in mitochondrial DNA are found in patients with all kinds of neurological and psychiatric disorders. Nevertheless,
a single genetic change that lowers the occurrence of mutations in mitochondrial DNA raises the odds that its carriers live to a hundred [...]. All this genetic variant might be doing is reduce by a tiny amount the rate of leakage of free radicals, a nearly imperceptible benefit that may mount up over a lifetime [...]. A convincing argument has indeed been made that, just by slowing down such a leakage, all degenerative diseases linked to aging could be postponed [...]. Note that subtle increases in the occurrence of the most common pathogenic mitochondrial DNA mutation within cells can precipitate abrupt shifts in the expression of nuclear genes—with a lower mutation level manifesting as diabetes or autism, a higher one as multisystem degenerative disease [...]. A high mutation level creates in fact a gene expression profile strikingly similar to that of Alzheimer’s and Parkinson’s diseases [...].
Finally, the imprinted brain theory epitomizes Bressan and Kramer's concluding suggestion that:
... the conventional notion that the cause of problems with the brain should be searched in the brain is naive as well as misleading [...]. It seems likelier that many such problems are driven instead by systemic defects to which the high-maintenance brain is especially sensitive—defects related to the generation and distribution of energy [...].
References
Bressan, P. and P. Kramer, Mental health, mitochondria, and the battle of the sexes. Biomedicines, 2021. 9: p. 116.


