Menopause
Menopause Revisited: Why Don’t Men Die at 50?
New evidence prompts a fresh look at the origins of menopause.
Posted June 14, 2020

My previous blog post reported that menopause has now been recognized in four whale species—killer whale, short-finned pilot whale, beluga, and narwhal—along with Asian elephants. I shall now focus on human menopause in light of this expanded evidence. Similarities but also striking differences exist.
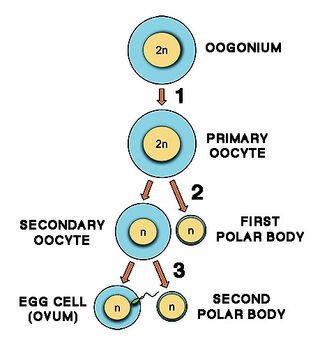
Do ovaries simply run out of eggs?
Menopause is directly connected with the exhaustion of the stock of tiny starter cells called primary oocytes in a woman’s ovaries. A thin layer of cells surrounds each oocyte, forming a primordial follicle.
All female mammals, including women, share a highly unusual feature: At birth, the ovaries contain a lifetime’s supply of starter follicles produced in the fetus. Following birth, no new follicles are produced. After puberty, in every cycle, a batch of starter follicles begins to mature. In humans and other mammals with single offspring, only one follicle proceeds to ovulation.
A 2010 paper by Hamish Wallace and Thomas presented the first-ever overview of the stock of follicles in human ovaries from conception through to menopause. By mid-pregnancy, a female fetus has a maximum of 300,000 starter follicles in each ovary, which largely persist until birth. Following birth, average numbers of primordial follicles per ovary decline inexorably, falling to 180,000 by the first menstruation (menarche) at around 13 years, to 65,000 by age 25, and 16,000 by age 35. By age 50, the average number of starter follicles per ovary has fallen to around 1,000. This number is widely accepted as the threshold for menopause.
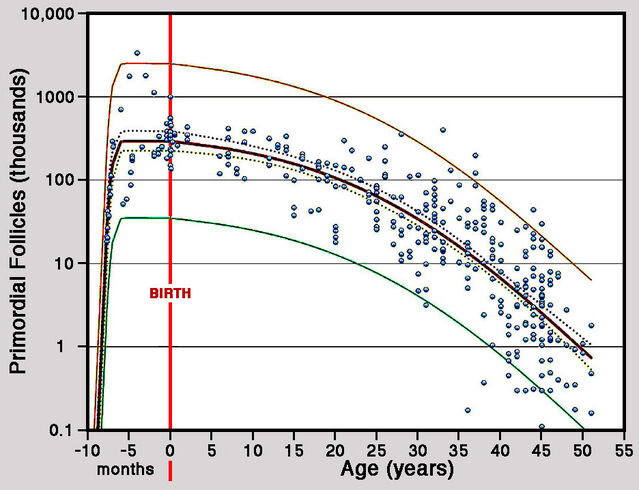
Is menopause connected to menarche?
If menopause is simply a result of ovaries running out of eggs, it should be linked with menarche. When menstrual cycles begin early, starter follicles should be depleted sooner, triggering early menopause. My 2014 blog piece, "The Second Curse on Women: Menopause," cited findings from Alan Treloar’s classic 1974 paper. Continuous menstrual histories recorded by over 300 women from student days through to natural menopause revealed that age at menopause (average: 50 years) was not correlated with age at menarche (average: 14 years).
Subsequent studies confirmed Treolar’s finding, a notable example being a 2018 paper by Elisabeth Bjelland and colleagues examining records for over 300,000 Norwegian women. Median age at menopause was 51, largely regardless of age at first menstruation. At the extremes, however, women with menarche at age 16 years and above did experience menopause a year later on average than women with menarche at or below age 13.
Overall, however, delayed menopause did not offset late menarche. Instead, the number of reproductive years decreased significantly as age at menarche increased. The lack of association between ages at menarche and menopause points to distinct control mechanisms.
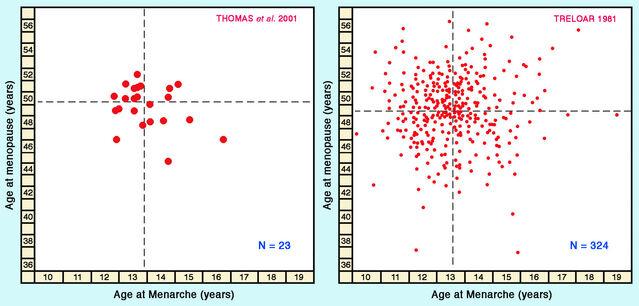
Whereas the relationship between ages at menarche and menopause has often been studied within human populations, comparisons across populations are rare. One exception is a broad international review by Frédéric Thomas and colleagues in 2001, examining ages at menarche and menopause for 23 countries. The average age of menopause across populations was close to 50. Figures from Thomas and colleagues also show no significant correlation between ages at menopause and menarche across populations.
Is menopause a byproduct or an adaptation?
Some authors propose that menopause did not evolve under natural selection but is merely a side-effect of extended human longevity. Others maintain instead that menopause evolved as an evolutionary adaptation. Closer examination, in fact, indicates that the abrupt termination of fertility in women is not a mere byproduct but an outcome of natural selection.
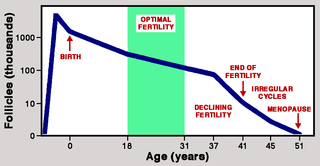
A 1992 paper by Malcolm Faddy and colleagues provides crucial evidence derived from the mathematical modeling of declining numbers of starter follicles as women’s ovaries age. They identified two stages, with a steady rate of loss during Phase 1 accelerated during Phase 2. The transition between phases occurred at about 38 years when starter follicles had fallen to 25,000 per ovary. More rapid loss during Phase 2 reduced the follicle count to 1,000 by age 51. Faddy and colleagues calculated that without accelerated loss in Phase II, this threshold would be reached some 20 years later.
Faddy and colleagues refer to the pattern of follicle loss from the human ovary as “programmed disappearance,” and more rapid loss during Phase 2 can be seen as a sign of natural selection acting to ensure that menopause begins near the age of 50. Although it is conceivable that the two-phase pattern has a different explanation, this seems highly unlikely. A 2015 paper by Christine Cloutier and colleagues compared the decline in starter follicles in chimpanzees with that in humans and found a striking difference: Contrasting with the steeper decline in follicle counts seen in the human ovary after age 38, the decline in chimpanzees showed a uniform pattern throughout.
A similar uniform pattern of decline is also seen in other primates, such as macaques and squirrel monkeys. This clearly supports the inference that natural selection drove the increased rate of follicle loss in humans.
Why did selection drive human menopause?
Menopause is clearly not a byproduct, but several alternative hypotheses have been proposed to explain it as an adaptation. The most widely cited is the "grandmother hypothesis," advocated by Kristen Hawkes and colleagues, according to which post-menopausal women promote the survival of grandchildren rather than producing more children of their own. Some studies have yielded results compatible with predictions from the grandmother hypothesis, while others have not. All studies face the problem that correlations derived from empirical observations often do not reflect causation.
A core problem with correlations is that additional factors not included in the analysis—confounding variables—may have a decisive influence. In any evaluation of the grandmother hypothesis, one potential confounder must surely be grandfathers. Yet an influential 1997 paper by Hawkes and colleagues, presenting a detailed analysis of the rôle of grandmothers among Hadza hunter-gatherers in Tanzania, contained not one single mention of the word “grandfather”!
Admittedly, some studies have reported that grandfathers do not demonstrably influence the survival of grand-offspring. But here’s the thing: Evolutionary hypotheses concerning menopause address the basic expectation that individuals should continue breeding more-or-less until they die. The grandmother hypothesis was conceived as a special-case proposal that grandmothers can disseminate their genes more effectively by enhancing the survival of their grandchildren than by continuing to produce children themselves. However, with strict monogamy, once a grandmother has become infertile, her husband will be effectively infertile as well. Surely grandfathers should also contribute to the survival of grandchildren to promote the dissemination of their genes?
We take it for granted that, as with men and women, male and female lifespans are similar. Yet if evolution led to women becoming infertile in mid-life because grandmothers can propagate their genes more successfully by nurturing grandchildren, whereas grandfathers do not contribute significantly to their survival, why don’t men die at 50?
Now it might be thought that lifespans in males and females must be similar because they share very similar genomes. But consider killer whales, one of five other mammal species currently known to have mid-life menopause resembling that of women. So far, only killer whales have been investigated in detail, yielding some interesting findings compatible with the grandmother hypothesis. However, a stunning difference from humans has passed almost unnoticed: Female killer whales reportedly have an average life expectancy of 46 years and maximum longevity of around 80. In males, by contrast, an average life expectancy is only 31 years, with a maximum close to 65. Survival beyond 50 is a rare occurrence. So we really do need to ask why men don’t die at 50.
The occurrence of menopause may be attributable, at least in part, to natural selection acting in an entirely different way. Think back to that unusual feature of mammal ovaries, with a lifetime’s stock of starter follicles at birth. Mutations occur predominantly during cell division and are much rarer with resting cells. The special pattern of follicle production in the mammal ovary, with minimized call division, reins in the build-up of mutations.
Recent studies have shown that mutations accumulate in human eggs at only a quarter of the rate seen with sperms. Moreover, all six mammals so far known to have menopause—women, four whale species, and Asian elephants—become infertile around age 50. Could this be an upper limit where accumulated mutations become simply too harmful for successful reproduction?
References
Allman, J., McLaughlin, T. & Hakeem, A. (1993) Brain weight and life-span in primate species. Proceedings of the National Academy of Sciences U.S.A. 90:118-122.
Alvarez, H.P. (2000) Grandmother hypothesis and primate life histories. American Journal of Physical Anthropology 113:435-450.
Austad, S.N. (1994) Menopause: An evolutionary perspective. Experimental Gerontology 29:255-263.
Bjelland, E.K., Hofvind, S., Byberg, L. & Eskild, A. (2018) The relation of age at menarche with age at natural menopause: a population study of 336,788 women in Norway. Human Reproduction 33:1149-1157.
Cloutier, C.T., Coxworth, J.E. & Hawkes, K. (2015) Age-related decline in ovarian follicle stocks differ between chimpanzees (Pan troglodytes) and humans. Age 37, Art. No. 10:1-9.
Faddy, M.J., Gosden, R.G., Gougeon, A., Richardson, S.J. & Nelson, J.F. (1992) Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Human Reproduction 7:1342-1346.
Hawkes, K., O'Connell, J.F. & Blurton Jones, N.G. (1997) Hadza women's time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Current Anthropology 38:551-577.
Sear, R. & Mace, R. (2008) Who keeps children alive? A review of the effects of kin on child survival. Evolution & Human Behavior 29:1-18.
Shanley, D.P., Sear, R., Mace, R. & Kirkwood, T.B.L. (2007) Testing evolutionary theories of menopause. Proceedings of the Royal Society of London B 274:2943-2949.
Thomas, F., Renaud, F., Benefice, E., de Meeüs, T. & Guegan, J.-F. (2001) International variability of ages at menarche and menopause: patterns and main determinants. Human Biology 73:271-290.
Treloar, A.E. (1974) Menarche, menopause and intervening fecundability. Human Biology 46:89-107.
Wallace, W.H.B. & Kelsey, T.W. (2010) Human ovarian reserve from conception to the menopause. PLoS One 5(1),e8772:1-9.




