Intelligence
Neuroimaging, Cannabis, and Brain Performance & Function
A fastidious meta-review and synthesis of the existing research brings clarity.
Posted January 21, 2018 Reviewed by Davia Sills
"I think pot should be legal. I don’t smoke it, but I like the smell of it." —Andy Warhol
Cannabis contains various molecules which bind to receptors in the brain, aptly called "cannabinoid receptors." Familiar ligands (which bind to those receptors) include THC (tetrahydrocannabinol) and CBD (cannabidiol), binding to receptors such as the CB1 and CB2 receptors with various downstream functions on the brain.
The primary neurotransmitter involved in innate (endogenous) cannabinoid activity is "anandamide," a unique "fatty acid neurotransmitter" whose name means "joy," "bliss," or "delight" in Sanskrit and related ancient tongues. This neurotransmitter system has only relatively recently been investigated in greater detail, and the basic biology is fairly well worked out (e.g., Kovacovic & Somanathan, 2014), improving understanding of therapeutic, recreational, and adverse effects of different cannabinoids, and paving the way for novel synthetic drug development.
The increasing interest in the therapeutic and recreational use of cannabis demands a greater understanding of the effects of cannabis on the brain and behavior. Because of the controversial and politicized nature of marijuana in societal discourse, strong beliefs about cannabis obstruct our capacity to have a reasoned conversation about the potential pros and cons of cannabis use and have impeded research initiatives. Nevertheless, many states have permitted the medical and recreational use of cannabis preparations, while the federal government is swinging back toward more restrictive policies.
The jury is out
Cannabis advocates, on the other hand, may paint too rosy a picture of the benefits of cannabis preparations, downplaying or dismissing relevant information about the hazards of cannabis in specific populations at risk for certain mental disorders, the risks of cannabis use disorders, and the negative effects of cannabis on certain cognitive processes accompanied by potentially deleterious, and even dangerous, effects on decision-making and behavior.
For instance, while cannabis preparations have been shown to be useful for pain management and functional improvement in various conditions, improving quality of life, cannabis may also cause errors in judgment and delays in information processing, which can lead not only to individual problems, but may get in the way of relationships and professional activities, even leading to possible harm to others by contributing to accidents.
Cannabis has been clearly associated with precipitating the onset of and worsening some illnesses, notably psychiatric conditions. Moreover, there is a growing interest in understanding the therapeutic and pathological potential of different compounds contained within cannabis preparations, most notably THC and CBD—although the importance of other components is increasingly recognized. For example, a recent study in the American Journal of Psychiatry strongly suggests that CBD, useful for treating intractable seizures (e.g., Rosenberg et al., 2015), may be of significant benefit as an augmenting agent for some with schizophrenia (McGuire at al., 2017).
The picture is not either-or, however. A deeper understanding of how cannabis affects different brain regions (under different conditions, e.g., acute vs. chronic use, with and without different mental illnesses and substance use disorder, with individual variations, etc.) is required to ground the debate in knowledge, and provide solid, reliable scientific findings to pave the way for future research. Foundational understanding is lacking, and while there is a growing body of research looking at various aspects of cannabis effects, as is always the case with an evolving body of research early on, the methodology has varied across many small studies, without a clear framework to encourage consistent approaches to investigation.
One question of obvious importance is: What are the effects of cannabis on key functional areas of the brain? How do functional and connectivity changes within key anatomic regions (“hubs,” in network theory) spread out to the brain networks in which they are central? How does cannabis use, to the extent we understand its effects, play on within specific tasks used to study cognition? What, in general, is the effect of cannabis on brain networks, including the default mode, executive control, and salience networks (three key networks in the densely interconnected “rich club” of brain networks)?
These and related questions are more important as we come to understand better how the mind/brain gap can be bridged by progress in mapping out the human neural connectome. The expectation is that increases or decreases in activity in different brain areas in users (compared with non-users) will correlate with broad changes across functional brain networks, which are reflected in patterns of differential performance on a large group of commonly used psychological research tools which capture different aspects of mental function and human behavior.
The current study
With this key consideration in mind, a multicenter group of researchers (Yanes et al., 2018) set out to collect and examine all the relevant neuroimaging literature looking at the effects of cannabis on the brain and on behavior and psychology.
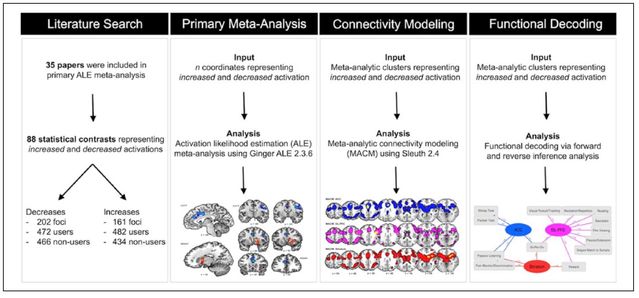
It’s worthwhile to review the meta-analytic approach used briefly and to discuss what kinds of studies were included and excluded, in order to contextualize and interpret the quite significant findings. They looked at literature including studies using fMRI (functional magnetic resonance imaging) and PET scans (positron emission tomography), common tools to measure indicators of brain activity, and conducted two preliminary assessments to organize the data.
First, they divided the studies into ones where activity in various brain areas was either increased or decreased for users versus non-users and matched up anatomic areas with the functional brain networks of which they are parts. In the second layer of refinement, they used “functional decoding” to identify and categorize different groups of psychological functions measured across the existing literature.
For example, studies look at a large but varying set of psychological functions to see how, if at all, cannabis changes cognitive and emotional processing. Relevant functions included decision-making, error detection, conflict management, affect regulation, reward and motivational functions, impulse control, executive functions, and memory, to provide an incomplete list. Because different studies used different assessments under different conditions, developing a pooled analytic approach is necessary to conduct a comprehensive review and analysis.
Searching multiple standard databases, they selected studies with imaging comparing users with non-users, with data available in the form of standard models suitable for pooled analysis, and which included psychological tests of perception, movement, emotion, thinking, and social information processing, in various combinations. They excluded those with mental health conditions, and studies looking at the immediate effects of cannabis consumption. They analyzed this curated data.
Looking at the convergence in neuroimaging findings across studies using ALE (Activation Likelihood Estimate, which transforms the data onto the standard brain mapping model), they identified which regions were more and less active. Using MACM (Meta-Analytic Connectivity Modeling, which employs the BrainMap database to compute whole-brain activation patterns), they identified clusters of brain regions which activated together.
They completed the functional decoding phase by looking at forward and reverse inference patterns to reciprocally link brain activity with mental performance, and mental performance with brain activity, to understand how different psychological processes correlate with functions in different brain regions.
Here is a summary of the overall meta-analytic "pipeline":
Findings
Yanes, Riedel, Ray, Kirkland, Bird, Boeving, Reid, Gonazlez, Robinson, Laird, and Sutherland (2018) analyzed a total of 35 studies. All told, there were 88 task-based conditions, with 202 elements related to decreased activation among 472 cannabis users and 466 non-users, and 161 elements regarding increased activation among 482 users and 434 non-users. There were three major areas of findings:
There were several areas of consistent (“convergent”) changes noticed among users and non-users, in terms of activation and deactivation. Decreases were observed in bilateral (both sides of the brain) ACCs (anterior cingulate cortex) and the right DLPFC (dorsolateral prefrontal cortex). By contrast, there was increased activation consistently observed in the right striatum (and extending to the right insula). It's important to note that these findings were distinct from one another, and this lack of overlap means they represent uniquely different effects of cannabis on different systems.
MACM analysis showed there were three clusters of co-activated brain regions:
- Cluster 1 — ACC included whole-brain activation patterns, including connections with the insular and caudate cortex, medial frontal cortex, precuneus, fusiform gyrus, culmen, thalamus, and cingulate cortex. The ACC is key for decision-making and processing conflict and is involved with exploring and committing to a given course of action (e.g., Kolling et al., 2016), and these related areas cover a broad range of functions related to the ACC. The insula is involved with self-perception, a notable example being a visceral experience of self-disgust.
- Cluster 2 — DLPFC included co-activation with parietal regions, orbitofrontal cortex, occipital cortex, and fusiform gyrus. As the DLPFC is involved with important executive functions, including regulating emotions, the experience of mood, and direction of attentional resources (e.g., Mondino at al., 2015) as well as aspects of language processing, and the related areas address key functions, including social information processing, impulse control, and related.
- Cluster 3 — Striatum included whole-brain involvement, notably the insular cortex, frontal cortex, superior parietal lobule, fusiform gyrus, and culmen. The striatum is involved with reward—the so-called “dopamine hit” referenced so often—which when properly regulated allows us to pursue optimal success, but in states of under-activity leads to inaction, and in excess contributes to addictive and compulsive behaviors. The evidence reviewed in the original paper suggests that cannabis use may prime reward circuits to predispose toward addiction, and possibly blunt motivation for ordinary activities.
While these clusters are functionally distinct in terms of how they are affected by cannabis, they overlap anatomically and spatially, highlighting the crucial importance of viewed brain activity from the connectome, networked point of view in order to grasp the translation of reductive brain findings to how the mind works, and how this plays out for people in day-to-day life.
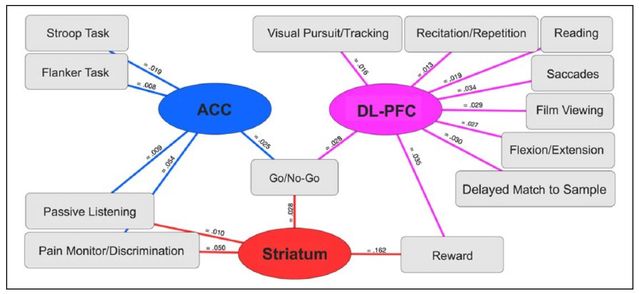
The functional decoding of the three clusters showed patterns of how each cluster correlates with a group of psychological tests: for example, the Stroop test, go/no-go task which involves fast decisions, pain monitoring tasks, and reward-assessing tasks, to name a few. I won’t review them all, but the findings are relevant, and some of them stand out (see below).
This overview of the cluster-task relationships is useful. Especially notable is the presence of the go/no-go task condition in all three functional areas:
Further considerations
Taken together, the results of this meta-analysis are profound and achieve the goals of focusing in on and distilling findings across the relevant literature investigating the effects of cannabis use on brain activation in populations without mental illness, looking at increased and decreased activity in localized brain regions, distributed clusters of distinct relevance, and the impact on key psychological processing tasks and function.
Cannabis lowers activity in both ACC and DLPFC clusters, and for people with normal brain function, this could lead to problems in executive function and decision-making. Cannabis is likely to cause inaccuracy in error monitoring, leading to misperception and performance issues due to mistakes, and may impede function during high-conflict situations, from both errors in judgment as well as from altered decision-making and subsequent execution. Decreased DLPFC activity could lead to emotional regulatory problems, as well as decreases in memory and reduced attentional control.
For people with psychiatric and medical conditions, the same brain effects could be therapeutic, for example reducing pain burden by decreasing ACC activity, alleviating traumatic memories and suppressing post-traumatic nightmares, treating anxiety with few side effects, or reducing psychotic symptoms (McGuire, 2017) by inhibiting activity in involved brain areas.
But cannabinoids also may trigger pathology, precipitating depression or psychosis, and other conditions, in vulnerable populations. Cannabis use also causes problems for the developing brain, leading to undesirable long-term effects (e.g., Jacobus and Tappert, 2014), such as reduced neurocognitive performance and structural changes in the brain.
Cannabis was shown, in contrast, to increase activity in the striatum and related areas generally. For people with normal baseline activity, this could lead to the priming of reward circuits, and as has been observed in numerous studies, could increase the risk of addictive and compulsive behaviors, predisposing to some forms of pathology. This amplification of reward activity (combined with effects on the first two clusters) may contribute to the "high" of marijuana intoxication, enhancing enjoyment and creative activity, making everything more intense and engaging, temporarily.
The authors note that all three clusters involved the go/no-go task, a test situation requiring the inhibition or performance of a motor action. They note:
"Here, the fact that distinct region-specific disruptions were linked with the same task classification may be indicative of a cannabis-related compound effect manifest across studies. In other words, a diminished capacity to inhibit problematic behaviors may be linked to concurrent reduction of prefrontal activity (ACC and DL-PFC) and elevation of striatal activity."
For some patients, cannabis reportedly alleviates symptoms of depression, characterized by core experiences of loss of enjoyment, excessive negative emotional states, and lack of motivation, among other symptoms, but heavier users are at increased risk for worsening depression (Manrique-Garcia et al., 2012).
However, in addition to potentially priming for addiction to other chemicals and enhancing experiences for those who enjoy being intoxicated with marijuana (others find it produces dysphoria, anxiety, unpleasant confusion, or even paranoia), users may find that in the absence of cannabis use, they are less interested in regular activities when they are not high, leading to decreased enjoyment and motivation.
These effects are different depending on several cannabis use-related factors, such as the timing and chronicity of use, as well as the type of cannabis and relative chemistry, given variations among different species and strains. While this study was not able to distinguish between the effects of THC and CBD, as data were not available on concentrations or ratios of these two key components in cannabis, it is likely that they have different effects on brain function which require further investigation to sort out therapeutic potential from recreational and pathological effects.
This study is a foundational study, setting the stage for ongoing research on the effects of various cannabinoids on the brain in health and illness, and providing important data to understand the therapeutic and damaging effects of different cannabinoids. The elegant and painstaking methodology in this study shines a spotlight on how cannabis affects the brain, providing significant data about the overall effects on brain networks as well as on cognitive and emotional function.
Questions of interest include the additional mapping of brain networks and correlating these findings with existing models of the mind, looking at the effect of different types of cannabis and patterns of use, and investigating the effect of cannabinoids (naturally-occurring, endogenous, and synthetic) for therapeutic purposes in different clinical conditions, recreational use, and potentially for performance enhancement.
Finally, by providing a coherent framework for understanding the existing literature inclusive of positive and negative effects of cannabis on the brain, this paper centers cannabis research more squarely in the mainstream of scientific study, providing a neutral, de-stigmatized platform to permit the debate on cannabis to evolve in more constructive directions than it historically has.
References
Mondino M, Thiffault F & Fecteau S. (2016). Does non-invasive brain stimulation applied over the dorsolateral prefrontal cortex non-specifically influence mood and emotional processing in healthy individuals? Front Cell Neurosci. 2015; 9: 399. Published online 2015 Oct 14.
Kolling TE, Behrens TEJ, Wittmann MK & Rushworth MFS. (2016). Multiple signals in anterior cingulate cortex. Current Opinion in Neurobiology, Volume 37, April 2016, Pages 36-43.
McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Tylor A, & Wright S. (2015). Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Neurotherapeutics. 2015 Oct; 12(4): 747–768. Published online 2015 Aug 18.
Rosenberg EC, Tsien RW, Whalley BJ & Devinsky O. (2015). Cannabinoids and Epilepsy. Curr Pharm Des. 2014; 20(13): 2186–2193.
Jacobus J & Tapert SF. (2017). Effects of Cannabis on the Adolescent Brain. Cannabis Cannabinoid Res. 2017; 2(1): 259–264. Published online 2017 Oct 1.
Kovacic P & Somanathan R. (2014). Cannabinoids (CBD, CBDHQ and THC): Metabolism, Physiological Effects, Electron Transfer, Reactive Oxygen Species and Medical Use. The Natural Products Journal, Volume 4, Number 1, March 2014, pp. 47-53(7).
Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T & Allebeck P. (2012). Cannabis use and depression: a longitudinal study of a national cohort of Swedish conscripts. BMC Psychiatry201212:112.




