
Depression
Bringing Joy Back to Childbirth
The development of a new drug may help with postpartum depression and its stigma
Posted August 25, 2019 Reviewed by Matt Huston
In the US. alone, 3 million new moms suffer from postpartum depression, or PPD.
Most moms agree that, despite the discomfort during pregnancy and the pain during labor, childbirth is a joyful experience. And I imagine that must be true as many women around the world have more than one child.
However, postpartum depression may take away some of the joy of seeing the face of your child.
According to the National Institute of Mental Health, PPD is characterized by overwhelming feelings of sadness and anxiety experienced by a new mom, which may compromise her ability to take proper care of her newborn or develop the maternal bond. Often, the affected mom is seen as guilty of negligence rather than someone who needs help. The stigma surrounding PPD has made it very difficult for patients to come forward and identify the threat to their health. At the same time, research has moved slowly.
But finally, very recently the FDA approved the first treatment for PPD.
The Brain in Postpartum Depression
PPD shares symptoms with classic depression (classic meaning the type of depression that is not specifically affecting moms who recently gave birth). Anxiety, sadness, and withdrawal from social and enjoyable activities are common among patients who suffer from depression. In the case of PPD, emotional detachment from the baby and strong self-doubt of one’s ability to actually care for the baby are in addition to an already distressed mental state. Frequently PPD patients have reported having thoughts of self-harm and even harming the baby. All this contributes to a strong feeling of guilt in the new mom, who sees herself as incapable of performing or even caring for the well-being of her newborn.
PPD is caused by a variety of factors including genetic predisposition and drastic hormonal changes during pregnancy and after labor. Looking after a newborn comes with sleep deprivation and can lead to extreme fatigue. In addition to the emotional and physical pain that the new mom may find herself in, it creates the perfect breeding ground for depression.
The cause(s) of PPD are unclear, but some research points to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, defects in GABA (responsible for the inhibitory signaling in the brain), and a decrease in a hormone called allopregnanolone. The HPA axis is known to be overactive during depression. In depressive states, it is also known that type A GABA receptors (a particular type of receptor for the inhibitory neurotransmitter GABA) are defective, and this has been pinpointed as the cause for the HPA axis hyperactivity during depression. In the case of PPD, alterations in the levels of allopregnanolone seem to be the trigger for the dysregulation of GABA and the HPA axis.
Allopregnanolone levels are very high during pregnancy, and they drop dramatically right after childbirth. This massive decrease in allopregnanolone can cause the dysfunction of GABA, which in turn messes up the HPA axis.
Before recently, the available treatments for depressed new moms were limited to SSRIs (selective serotonin-reuptake inhibitors), the common medication for classic depression. However, SSRIs have proven time after time ineffective in most cases of PPD. Now, there is a newly FDA-approved drug that could represent a turning point in the treatment of PPD: Brexanolone.
A New Drug, a New Hope
Brexanolone is a drug that mimics a soluble form of allopregnanolone. Formerly known as SAGE-547, this drug was also studied in the treatment of a particularly hard-to-treat form of epilepsy (called super-refractory status epilepticus), due to its effects on the modulation of GABA. In moderate and severe cases of PPD, brexanolone can modulate type A GABA receptors. Potentially, brexanolone could be also an effective treatment for major depression disorder, but so far, it has only been tested in the context of PPD.
The clinical trial for brexanolone against PPD was spread out across the country in 30 different clinical facilities, where women of ages between 18 and 45 suffering from moderate to severe PPD were treated. Some got the drug, some got a placebo.
Women who were treated with brexanolone already felt less depressive symptoms as early as 24 hours after the first intravenous injection of the drug. Most of the patients improved significantly after 60 hours of treatment, with effects that lasted even 30 days after, with no relapse. Admittedly, women who were treated with placebo instead also experienced improvements in their emotional health, but to a lesser degree.
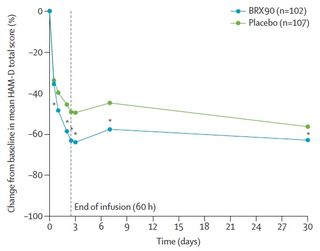
Brexanolone, in view of the positive outcomes in clinical trials, was finally approved in March by the FDA (USA Food and Drug Administration) under the name Zulresso. Sage Therapeutics, who will be commercializing this drug, has already seen the benefits of this drug from a financial point of view.
Zulresso is administered as a continuous intravenous infusion for 60 hours, the length of treatment that was proven to be most effective during clinical trials. During the treatment, the FDA recommends that the moms are accompanied by their newborns.
Though Zulresso represents a new hope for moms suffering from PPD, it also has some downsides. A treatment of this length (60 hours or 2 days and a half) and with the associated risks of a sudden loss of consciousness and excessive sedation, it requires constant monitoring of the patient in a medical facility which makes the cost of treatment incredibly high. At the moment, it is estimated that a full treatment with Zulresso will come up to more than $30,000. It goes without saying that this will deter women and families with tight budgets, and not all insurance companies will be covering this kind of treatment.
Overcoming the Stigma
Although the duration and especially the cost of treatment may make this new hope for PPD unattainable to many patients, the fact that a drug has been approved by the FDA to treat PPD represents a major step forward and a push for further PPD research.
There is a lot of stigma surrounding PPD. Moms suffering from PPD are sometimes seen as insensitive, careless, and at blame for their negligence, but these are all caused by the underlying condition PPD. PPD is not a weakness, nor a flaw in the mom’s character. It is a health issue that needs to be addressed for the sake of the mom and the baby. Hopefully, with this new treatment at hand, PPD can be addressed more effectively. With better and more efficient diagnosis, we can stop classifying women suffering from PPD as “bad moms” and recognize PPD as what it really is: a significant health problem that needs to be treated.
This article was originally published in NeuWrite San Diego. Also available in Spanish.
References
Samantha Meltzer-Brody, et al. Brexanolone injection in postpartum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials.The Lancet, Volume 392, Issue 10152, 2018, Pages 1058-1070, ISSN 0140-6736, https://doi.org/10.1016/S0140-6736(18)31551-4.
Samuel T. Wilkinson and Gerard Sanacora. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discovery Today, Volume 24, Issue 2, 2019, Pages 606-615, ISSN 1359-6446, https://doi.org/10.1016/j.drudis.2018.11.007
Shen, Qiuying et al. “gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression.” Biological psychiatry vol. 68,6 (2010): 512-20. doi:10.1016/j.biopsych.2010.04.024



