Vagus Nerve
Guts, Wits, Microbiome, and the Vagus Nerve
Specific gut microbiome may influence social behaviors via the vagus nerve.
Posted January 21, 2019
In 2017, I wrote a nine-part series called, “A Vagus Nerve Survival Guide to Combat Fight-or-Flight Urges.” Since then, I’ve kept my antennae up for any new research about how the gut-brain axis relies on the vagus nerve to send microbiome-based messages that affect cognition and other aspects of behavior from the gut to the brain. (See "Does Gut Microbiome Influence Mindset and Mental Toughness?" and "Vagus Nerve Facilitates Guts, Wits, and Grace Under Pressure.")
Suffice to say, I was thrilled to read a recent press release, "The Power of the Microbiome: A Microbe-Based Treatment Reverses Social Deficits in Mouse Models of Autism," about a new study published this week in the journal Neuron which found that administering a microbe called Lactobacillus reuteri rescues social impairment in mice with autism-like behaviors via the vagus nerve.
The authors of this study (Sgritta et al., 2018) were pleasantly surprised to discover that L. reuteri appears to trigger the recovery of social deficits in all of the autism spectrum disorder (ASD) mouse models they tested. These preliminary findings indicate that someday a microbiome-based approach could be implemented to improve social functioning in people diagnosed with ASD.
The lead author of this study, Martina Sgritta, is a postdoctoral associate in the lab of senior author Mauro Costa-Mattioli, who is a professor in the Department of Neuroscience and the director of Baylor College of Medicine’s Memory and Brain Research Center.
This pioneering study offers fresh clues about how vagus nerve pathways facilitate a two-way communication superhighway that gut microbiome may utilize to send messages between the lowest viscera of the GI tract and the highest regions of the brain.
Previous research identified that the vagus nerve stimulates neurons in the brain to produce oxytocin. During healthy face-to-face interactions, oxytocin is thought to bind with receptors in a pleasure center of the human and mouse brain called the “ventral tegmental area” (VTA) and trigger feelings of reward that drive sociability.
For this study, Sgritta and her colleagues purposely disrupted vagus nerve connections between the gut and brain to identify if this interference would disrupt oxytocin from binding to VTA receptors and influence the ability of L. reuteri to rescue and restore social reward behaviors in ASD mice.
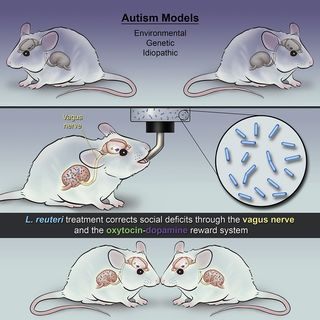
“We discovered that L. reuteri promotes social behavior via the vagus nerve, a nerve that bidirectionally connects the gut and the brain and the oxytocin-dopamine reward system,” Sgritta said in a statement. “Interestingly, we found that when the vagus nerve was severed and the brain-gut connection was disrupted, L. reuteri could no longer restore social behavior in ASD mice. In addition, when we genetically engineered mice to lack oxytocin receptors in reward neurons or blocked the receptors with specific drugs, the treatment with L. reuteri also failed to reverse the social deficits in the ASD mice.”
The announcement of this research from Baylor College of Medicine sums up the significance of this study, "Costa-Mattioli believes that these new findings strengthen the rather unconventional idea that it might be possible to treat specific neurological symptoms through the gut microbiome using specific bacterial strains."
A preview article, “Gut Microbes Join the Social Network” by Helen Vuong and Elaine Hsiao of UCLA (who were not involved in the new Baylor study) was published in the January 16 issue of Neuron. Vuong and Hsiao write, “The gut microbiome is increasingly implicated in the regulation of social behavior across model organisms. In this issue of Neuron, Sgritta et al. (2018) examine the role of the gut microbiome in social reward circuits and sociability in three mouse models of autism spectrum disorder."
The latest state-of-the-art vagus nerve and gut-microbiota-brain axis research from the Costa-Mattioli Lab might be a game changer. The findings by Sgritta et al. could lead to revolutionary treatments that could improve sociability for everyone who is touched by the social deficits associated with ASD. "We have begun to decipher the precise mechanism by which a microbe in the gut modulates brain function and behavior. This could be key in the development of new and more effective therapies,” Costa-Mattioli concluded.
References
Martina Sgritta, Sean W. Dooling, Shelly A. Buffington, Eric N. Momin, Michael B. Francis, Robert A. Britton, Mauro Costa-Mattioli. "Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder." Neuron (First published online: December 3, 2018) DOI: 10.1016/j.neuron.2018.11.018
Helen E. Vuong and Elaine Y. Hsiao. "Gut Microbes Join the Social Network." Neuron (First published: January 16, 2019) DOI: 10.1016/j.neuron.2018.12.035


