Coronavirus Disease 2019
COVID-19: Mitochondria’s Pivotal Role -Revision
Part 3: Mitochondria protect us from COVID-19, even death.
Posted June 16, 2020
Symptoms such as sleep disturbance, fatigue, appetite disturbance, loss of interest in usual activities and decreased social interaction are observed in both sickness behavior and clinical depression related to viral infection (1). Loss of energy, loss of motivation, chronic unremitting fatigue, with or without muscle weakness and excessive sleeping or hypersomnia are cardinal symptoms of mitochondrial distress. Fatigue and related symptoms are often misdiagnosed as depression. Major depression may be the clinical expression for reduced mitochondrial respiration and impaired mitochondria-regulated immune response (2).
Our mitochondria are the energy powerhouses of the cell. They could accurately be compared to armored nuclear power plants because they activate and safely contain (when healthy) the intense explosions required to cleave the high-energy bond between two oxygen atoms. These oxygen-cleaving explosions would cause devastating, widespread oxidative damage (akin to a nuclear meltdown) if they occurred anywhere else in the body. This catabolic oxygen combustion helps break down food (carbohydrates, proteins, and fats) and converts it into energy units called ATP (adenosine triphosphate),… The key point here is that mitochondria are exponentially better at producing ATP… and any physiological process that causes mitochondrial dysfunction can cause widespread cellular energy deficits and fatigue, adversely affecting virtually all organ systems including the brain (3).
Hypertension, one of the main risk factors for SARS-COV and SARS-COV-19, is “prominently associated with the loss of cardiolipin, a phospholipid uniquely found in the inner mitochondrial membrane and necessary for it’s proper formation… Furthermore, cardiolipin regulates mitochondrial dynamics and prevents the formation and opening of the mitochondrial permeability transition pore (mPTP), and release of cytochrome C from mitochondria to the cytosol (aqueous solution inside of the mitochondria) where it triggers apoptosis” (4). In other words, hypertension is part of a process whereby a hole is opened in the “armored nuclear power plant” of the mitochondria that can then be pierced and destroyed by SARS-COV-19.
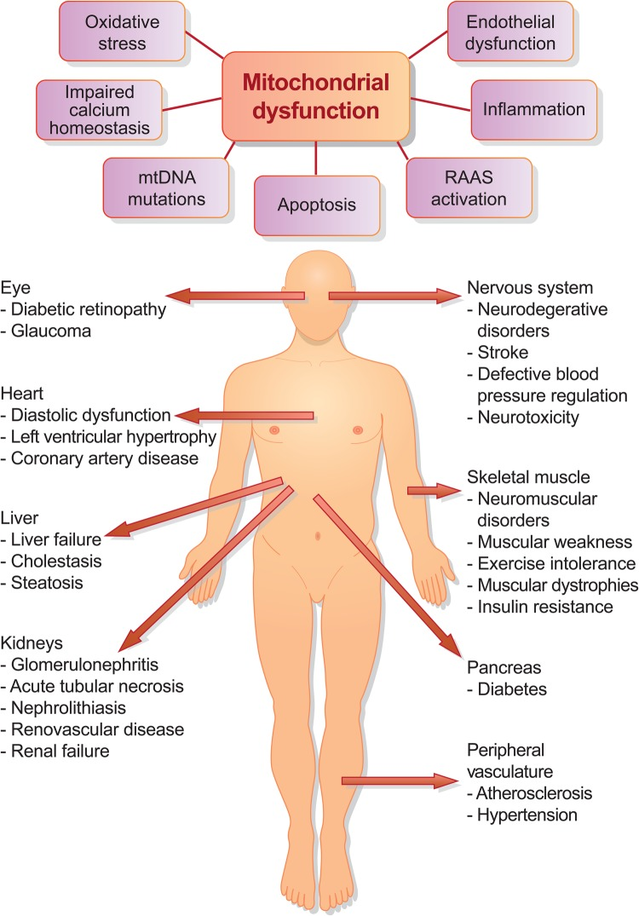
Figure 1: (A) Schematic of proposed mitochondria-mediated mechanisms of disease progression. (B) Mitochondrial damage and dysfunction have been implicated in pathological conditions in many organs (4).
Mitochondrial dysfunction can be caused by viral infection including SARS-COV and its individual viral protein 3a which results in mitochondrial death or apoptosis (5). In cellular homeostasis or health, there is a balance between the BCL-2 family protein, which are neuroprotective and Bax proteins which can be transformed to act like Darth Vader (death proteins) and can set off a cell-death cascade. This can occur in response to extracellular stimulation by stress, virus infection and by immune cytokines secretions which generally guide a healthy immune response. Bax exist in one type of relatively stable molecular form in the cell’s cytoplasm, but with viral infection it changes form along with increased levels of tumor suppressor protein P53. Studies reveal Bax moves to the outer membrane of the mitochondria where it inserts itself. This causes the release of the cytochrome C, thus initiating apoptosis or cellular death (5) with the release of the mitochondrial DNA eventually into the blood stream.
The link between mitochondrial dysfunction, obesity and Type 2 Diabetes has been discussed (6). Insulin resistance in skeletal muscle is a major hallmark of type 2 diabetes mellitus (T2D) and obesity and both have been linked to decreased muscle mitochondria reproduction and their dysfunction. The uncoupled mitochondrial state is necessary for increased ATP synthesis. Excessive mitochondrial coupling is a central expression of mitochondrial “dysfunction in obesity that may contribute to the development of metabolic pathologies such as insulin resistance and diabetes” (7). Non-alcoholic fatty liver disease is obesity related and excessive liver fat accumulation damages mitochondria.
A pertinent clinical example MD is the 53 years old Italian woman described in Science (8) with “classic symptoms of a heart attack”, but with no blocked coronary arteries. Nevertheless, her left ventricle (i.e. the strongest chamber in the heart) “was so weak that she could only pump her blood at one-third of its normal volume.” The latter could suggest impaired mitochondrial integrity with compromised energy production. Most of the risk factors for COVID-19 are found in Figure 1, and they are interrelated in that they all have relative degrees of impaired MD. One of the only exceptions not yet discussed is age (9).
Mitochondria deteriorate with age, losing respiratory activity, accumulating damage to their DNA (mtDNA), and producing excessive amounts of reactive oxygen species (ROS)… The hypothesis that aging is caused by accumulating ROS damage is supported by the inverse correlation between mitochondrial ROS production and life span in mammals…. One of the concerns that arises with aging is the increased susceptibility to infections. In future research on treating immunological deficits, a mitochondria-centered approach may be in order. Beyond mediating apoptosis of infected cells, mitochondria are emerging as critical components of the innate immune response. It has been shown that the ATP needed for purinergic signaling (e.g. adenosine and ATP), T-cell regulation, and initial activation of neutrophils comes from mitochondria. ATP production and mitochondrial Ca2 + buffering are needed for antigen presentation and processing, and ROS are a part of the signaling pathway that activates inflammatory proteins. With the current rise of multidrug-resistant “superbugs”, other ways of combating infections grow increasingly crucial. Based on accumulating evidence, mitochondria may be viable therapeutic targets….
As mediators of immunity, mitochondria are consequently targeted by several viruses: Influenza… induces mitochondrial dysfunction as a mechanism of crippling the innate immune system response. The SARS virus also targets mitochondria. The virus-encoded protein ORF-9b localizes to mitochondria and triggers degradation of DRP1, MAVS, TRAF3, and TRAF6, thus evading the host immune responses.… (9).
The SARS virus localizes to physically distort and manipulate cellular mitochondrial function, thus enabling it to avoid/evade normal innate immunity defenses. Any virus has many modes of action which can change or mutate. Nevertheless, immune defense depends on energy production and thus the importance of mitochondrial integrity cannot be overlooked.
Immune cells, as with all cells, cannot function without healthy multiple mitochondria. Their ATP, the life source, is needed for example, in T cell regulation and the initial activation of neutrophils. Humans are more susceptible to infection with aging, and our immune T cells don’t respond as well to pathogens or vaccines as our mitochondria begin to malfunction. This is reflected in the age related cognitive, cardiovascular, physical, metabolic, etc. changes, experienced and observed. Nevertheless, poor T cell response might not only be the result of aging but may be part of the cause of aging by releasing excessive inflammatory cytokines (the cytokine storm). T cell mitochondria transcription factor A or TFAM is a DNA protein essential for the mitochondrial’s separate unique genome sequence and maintenance. When it is genetically modified (TFAM deficiency) to be energy inefficient, it forced the T cells from ATP use into a less efficient mode of energy production. These mice rapidly aged with deterioration in their functions noted previously. “T cell metabolic failure induces the accumulation of circulating cytokines, which resemble chronic inflammation characteristic of aging (“inflammaging”). This cytokine storm itself acts as a systemic inducer of senescence” (10). The importance of TFAM alterations (e.g. mutations, deletions, changes in expression) is also revealed in its key role in neurodegeneration including Parkinson and Alzheimers (11).
A major immune defense against viral infection necessary for cellular viability, is autophagy, (the body’s way of cleaning out damaged and dysfunctional cells, and recycling parts of them to regenerate new healthier cells). Autophagy delivers viral proteins and viruses to lysosomes for degradation. However, lysosomes are impaired by the loss of mitochondrial function (11) such as in SARS-COV and COV-19 related impairments. “Inflammaging”, and decreased autophagy accelerate the metabolic compromised state of people with known risk factors (e.g. cardiovascular, diabetes, obesity, age, hypertension, kidney impairment, etc.). It is no surprise that our young are more resilient since they generate sufficient ATP. Like a candle with decreasing oxygen, the lights dim and go out.
References
1. Okusaga, O., Yolken, RH., Langenberg, P., et al: Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011 Apr;130(1-2):220-5. doi: 10.1016/j.jad.2010.09.029.
2. Marrs, C.: Micronutrient deficiencies and mitochondrial dysfunction. Chap 7 in Integrative Therapies for Depression 2016, Greenblatt, JM., Brogen, K, editors CRC Press, Boca Raton, FL. Pgs 73 – 95.
3. Raffelock, D.: Mitochondrial dysfunction and physiological depression. Cap 6 in Integrative Therapies for Depression 2016, Greenblatt, JM., Brogan K, editors CRC Press, Boca Raton, FL. Pgs 61 – 72.
4. Eirin, A., Lerman, A., Lerman, L. O.: Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur Heart J. 2014 Dec 7; 35(46): 3258–3266. doi: 10.1093/eurheartj/ehu436
5. Padhan, K., Minakshi, R., Towheed MAB., Jameel, S.: Severe acute respiratory syndrome coronavirus 3a protein activates the mitochondrial death pathway through p38 MAP kinase activation. J Gen Virol. 2008 Aug;89(Pt 8):1960-1969. doi: 10.1099/vir.0.83665-0.
6. Højlund, K., Mogensen, M., Sahlin, K., Beck-Nielsen, H.: Mitochondrial Dysfunction in Type 2 Diabetes and Obesity. Endocrinology and Metabolism Clinics of N. America, Sept. 2008, 37, 3, 713-731
7. Arruda, A. P., Pers, B. M., Parlakgül, G., et al.: Chronic Enrichment of Hepatic Endoplasmic Reticulum-Mitochondria Contact Leads to Mitochondrial Dysfunction in Obesity. Nat Med 2014 Dec;20(12):1427-35. doi: 10.1038/nm.3735.
8. Wadman, M., Couzin-Frankel, J., Kaiser, J., Matacic, C.: How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science April 17, 2020.
9. Lane, RK., Hilsabeck, T., Rea, SL.: The role of mitochondrial dysfunction in age-related diseases. Biochim Biophys Acta. 2015 Nov;1847(11):1387-400. doi: 10.1016/j.bbabio.2015.05.021.
10. Desdín-Micó, G., Desdín-Micó, G., Desdín-Micó, J., et al.: T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 21 May 2020: eaax0860 DOI: 10.1126/science.aax0860
11. Baixuli, F., Acín-Pérez, R., Villarroya-Beltrí, C., et al.: Mitochondrial Respiration Controls Lysosomal Function During Inflammatory T Cell Responses. Cell Metab. 2015 Sep 1;22(3):485-98. doi: 10.1016/j.cmet.2015.07.020.




