
Genetics
The Epigenetics of Childhood Trauma
Part 1: How childhood adversity can provoke long-term health consequences.
Posted September 18, 2019 Reviewed by Gary Drevitch
This is the first of two posts regarding the epigenetics of childhood adversity, based on excerpts from my new book, This Is Depression.
To understand the term epigenetics, it’s necessary to have some background information about what genes are and how they work.
Humans are made with a built-in instructions book called deoxyribonucleic acid, or DNA, which is found in every cell in your body. Your DNA contains your genetic code, which provides the instructions necessary to grow, develop, reproduce, and stay alive. We keep our DNA wrapped around proteins and coiled into X shapes called chromosomes. We have 23 pairs of chromosomes, 46 in all. Twenty-two of those pairs look identical, whether we are male or female, but the twenty-third are sex chromosomes. Males have an X and a Y sex chromosome, while females have two X sex chromosomes.
A human being is created by the union of a single egg and a single sperm. The egg and sperm each contain a single copy of its owner’s DNA (23 chromosomes from each), which means the offspring receives one-half of their DNA from Mom and one-half from Dad. Identical twins have exactly the same DNA, but otherwise, every sibling receives a different mix of DNA from their parents. It truly is a genetic lottery. One sibling might be short with striking blue eyes, while the other is six feet tall with stinky feet. (In my family, we believe in the stinky feet gene!)
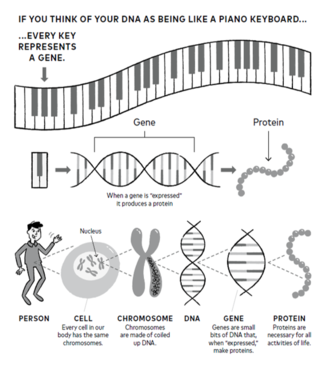
If every cell in your body contains the same DNA, how does a cell know to become a brain cell or a liver cell or a skin cell?
I think of DNA as being like a piano keyboard. Every person has their own unique keyboard (except identical twins; they have identical keyboards). Each key on your keyboard represents a section of DNA known as a gene. Genes hold the information needed for cellular differentiation: When specific genes are turned on (expressed) or turned off (suppressed) they direct what a cell will become. Specific genes make proteins and control traits like eye color or height, and other genes direct cells to become heart, brain, or skin cells.
DNA is surprisingly simple. It’s made up of just four chemicals: adenine (A), cytosine (C), guanine (G), and thymine (T). Genes are comprised of sequences of these four chemicals, which hold the instructions that direct a cell to produce specific proteins.
If our DNA is like a piano keyboard, the way the keys are played (the way genes are expressed) makes you who you are. Some keys are not played at all and others are always played. Some are played softly while others are played harshly. If, how, and when your genes are expressed ultimately makes you the unique individual you are. Think of it as “your song” or “the music of you.” Interestingly, your tune can change, and what causes that change is epigenetics.
Epigenetics is the science of gene expression. Your DNA is written in permanent marker; it can’t be changed or erased. Epigenetics is written in pencil: How our genes are expressed can change, thus we change our tune throughout our life. Epigenetics is the interface between nature (the genes you inherited from your parents) and nurture (your life experiences). How your genes are expressed, whether they are turned off or on, or played softly or harshly, depends on the type of genes you inherited from your parents, your developmental stage (e.g., puberty, menopause), and your environment.
Do epigenetic changes occur during normal development?
The changes that occur during puberty are a good example of a normal epigenetic cascade because some genes are expressed and others are suppressed, causing males and females to develop different secondary sexual characteristics. Of course, this is a complicated process involving hormones and other brain chemicals, but it’s all laid out in our instruction booklet—our DNA—and the genes that our DNA is comprised of.
The body changes that take place during menopause or pregnancy are other examples of normal epigenetic cascades that happen at specific times during our life. When these changes occur can be influenced by inherited biological factors as well as by our environment.
We now know that our inherited genetic code, as well as life events and our environment, can affect how our genes are expressed. Toxin-related epigenetic changes are an example of our environment impacting gene expression. The scary part of this story is that research has demonstrated that epigenetic changes related to toxins can be inherited by our children.
For example, we know that toxins found in cigarette smoke are associated with many types of cancer, by triggering the expression of cancer-causing genes or the suppression of genes that protect against cancer. If a father smokes and the toxins in the smoke provoke an epigenetic alteration in his genome, that altered gene can be passed on to his child. If the gene’s epigenetic change is activated in his child, it can provoke the development of lung cancer, even if the child is an otherwise healthy non-smoker who has never been exposed to any lung-cancer risk factors.
Research on schizophrenia provides several examples of gene-environment interactions that may trigger the development of the illness. Examples of potential triggers that can alter gene expression and might provoke the development of schizophrenia include infections during pregnancy, abnormal bowel flora (microorganisms that live in the gut), and smoking cannabis.
These and other environmental exposures are of greatest concern in vulnerable individuals, like those who have a family history of the disorder. Some families have specific genes in their DNA that likely constitute a risk for the disorder, and with exposure to a specific infection or cannabis, a gene that promotes schizophrenia might be activated, resulting in the onset of symptoms.
In my next post, I’ll share how childhood adversity can provoke epigenetic changes that may impact physical and mental health.
References
Danese, A., Moffitt, T.E., et al. (2009) “Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers.” Arch Pediatr Adolesc Med. 163; 12: 1135–43.
Lucassen, P.J., Oomen, C.A., et al. (2015) “Regulation of adult neurogenesis and plasticity by (early) stress, glucocorticoids, and inflammation.” Cold Spring Harb Perspect Biol. 7; 9: a021303.
Lucassen, P.J., Naninck, E.F., et al. 2013 “Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics.” Trends Neurosci. 36; 11: 621–31.
Lucassen, P.J., Pruessner, J., et al. (2014) “Neuropathology of stress.” Acta Neuropathol. 127; 1: 109–35.(2014), pp. 1509–1517).
Molet, J., Maras, P.M., Avishai-Eliner, S., Baram, T.Z. (2014) “Naturalistic rodent models of chronic early-life stress.” Dev Psychobiol. 127; 1 : 1675–88.
Naninck, E.F., Hoeijmakers, L., et al. (2015) “Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice.” Hippocampus 25; 3: 309–28.
Pan, P., Fleming, A.S., Lawson, D., et al. (2014) “Within- and between-litter maternal care alter behavior and gene regulation in female offspring.” Behav Neurosci 128; 6: 736–48. https://doi.org/10.1038/s41380-018-0039-z
Singh-Taylor, A., Korosi, A., Molet, J., et al. (2014) “Synaptic rewiring of stress-sensitive neurons by early-life experience: a mechanism for resilience?” Neurobiology of stress 1: 109–115.
Yam, K.Y., Naninck, E.F., Schmidt, M.V., et al. (2015) “Early-life adversity programs emotional functions and the neuroendocrine stress system: the contribution of nutrition, metabolic hormones and epigenetic mechanisms.” Stress 18; 3: 328–42.
Szutorisz H, Hurd YL. (2016) Epigenetic Effects of Cannabis Exposure. Biological Psychiatry. 79:586–594
Hosak L, Hosakova J. The complex etiology of schizophrenia– general state of the art. Neuroendocrinology Letters Volume 36 No. 7 2015
Ranganathan M, Skosnik PD, and D’Souza DC. (2016) Marijuana and Madness: Associations Between Cannabinoids and Psychosis. Biological Psychiatry. 79:511–513
Brown A, Fiori LM and Turecki G (2019) Bridging Basic and Clinical Research in Early Life Adversity, DNA Methylation, and Major Depressive Disorder. Front. Genet. 10:229. doi: 10.3389/fgene.2019.00229
Szutorisz H, Hurd YL. (2016) Epigenetic Effects of Cannabis Exposure. Biological Psychiatry; 79:586–594
Hosak L, Hosakova J. (2015) The complex etiology of schizophrenia– general state of the art. Neuroendocrinology Letters Volume 36 No. 7



