Sex
Sperm Selection: Fertilization Not Random After All?
From choosing a baby’s sex to natural mechanisms in the female genital tract.
Posted September 20, 2018 Reviewed by Ekua Hagan
For years, I seriously doubted that pre-selecting a baby’s sex was possible without radical intervention. Here is why: I well remember discussing this with a Swiss veterinary professor back in the 1980s. Matter-of-factly, he pointed out that so much money is at stake with domestic mammals that any practical solution would have been discovered long ago.
My previous post on influencing a baby’s sex (Boy or Girl: Couples Trying to Load the Dice: August 21, 2018) barely skimmed the surface. This vast subject has multiple ramifications. Many cases of marked bias in sex ratios at birth have been documented for both captive and free-living mammals, including primates. But no natural mechanism for selecting sperm before fertilization is known. It was hence widely assumed that biases arise after conception because of differences in survival between males and females in the womb. Nevertheless, some form of sperm selection before conception remains possible. Whereas an egg always has a single X-chromosome, a sperm bears either an X- or a Y-chromosome and therefore determines the offspring’s sex.
Sex pre-selection
My previous blog post homed in on the possibility that the timing of coitus relative to ovulation can influence a baby’s sex. In their still popular book, How to Choose the Sex of Your Baby, first published 40 years ago, Landrum Shettles and David Rorvik claimed that intercourse close to ovulation favours conception of a boy. They prescribed additional conditions supposedly favouring conception of either girls or boys. Importantly, Shettles’ recommendations rested on his conviction that sperms differ radically according to whether they bear a Y-chromosome (“Y-sperms”) or an X-chromosome (“X-sperms”). Because the Y-chromosome is distinctly smaller than the X, Shettles opined that Y-sperms swim faster. He also speculated that Y-sperms have shorter lives. Moreover, in a 1960 Nature paper he claimed that the heads of X- and Y-sperms differ distinctly in appearance under a phase contrast microscope. This was never confirmed.
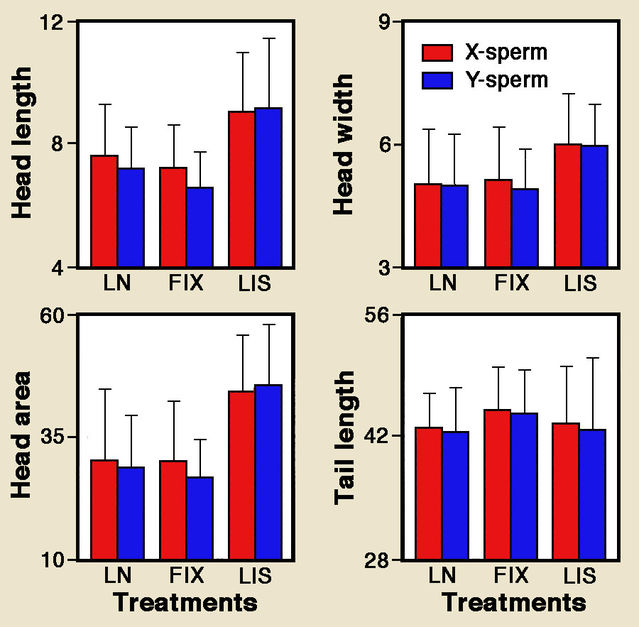

The amount of DNA in the nucleus does indeed differ between X- and Y-sperms. Remember, however, that human sex cells bear 23 chromosomes, only one of which is an X or Y. So the difference in total DNA between human X- and Y-sperms is marginal, less than 3%. Nonetheless, in a 1997 paper Ke-hui Cui reported that heads of human X- and Y-sperms differ significantly by about 5% in length, perimeter and area, and that X-sperms have significantly bigger necks and tails. Yet a later study by Amjad Hossain and colleagues documented considerable overlap between X- and Y-sperms with small, non-significant overall differences.
Separating X- and Y-sperms with density gradients
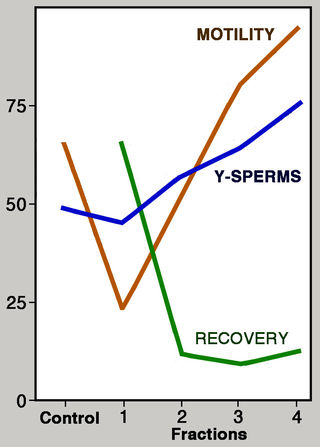
Attempts to separate X- from Y-sperms have a chequered history, beginning with a 1973 Nature paper by Ronald Ericsson and colleagues reporting successful enrichment of human Y-sperms. A reliable method for distinguishing X- from Y-sperms — crucial for assessing success — was seemingly provided by a report that Y-chromosomes can be specifically stained with quinacrine (originally used to combat malaria). Under fluorescence microscopy, the tip of the long arm of the Y-chromosome reportedly shows up as a bright dot (F-body). Ericsson and colleagues employed a separation method involving a vertical column of albumen with progressively increasing density from top to bottom, based on the idea that Y-sperms swim faster than X-sperms and therefore migrate more rapidly. The concentration of Y-sperms should accordingly increase from one layer to the next, and Ericsson and colleagues indeed reported that they were able to “isolate repeatedly up to 85% Y sperm”.
Soon afterwards, Ericsson patented the albumen method for sperm separation, franchised from 1975 through the company Gametrics Limited (of which he remains President). An additional procedure was designed for selecting X-sperm. Scores of clinics gradually joined an international network using the method under licence. Currently, approximate success rates of 83% and 78% for male and female selection with the Gametrics procedures are indicated on the internet.
Questions were, however, raised about the Ericsson method virtually from the outset. An initial impetus came from two independent papers published back-to-back in Nature in 1975, the first by J.M. Evans and colleagues and the second by Alan Ross and others. Both documented failed attempts to replicate the findings reported by Ericsson and colleagues in 1973. The two research teams repeated the methods as published but found no evidence of enrichment of human Y-sperms. Subsequently, many other authors reported similar lack of success.
Automatic sperm sorting
I distinctly remember my first encounter, some fifteen years ago, with the novel cell-sorting process of flow cytometry. While visiting the University of Southern California, I met Norman Arnheim, who explained his pioneering genetic analyses of single sperm cells. He proudly presented a large machine that ingeniously processed appropriately diluted semen samples to generate a stream of droplets, each usually containing a single sperm but sometimes two or none. As each droplet fell from a small nozzle, a beam deflected it horizontally according to the number of sperms present. Only droplets containing single sperms, deflected over a corresponding distance, were collected. Arnheim smilingly explained that operating the machine was deadly boring, so the manufacturer had thoughtfully built in a music center. It was, I believe, playing Wagner’s Ride Of The Valkyries.
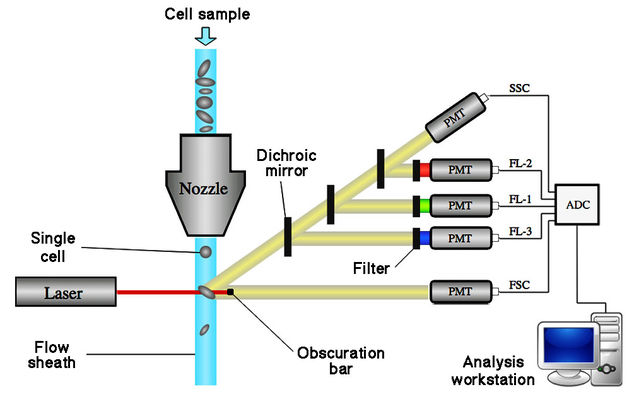
Only later did I discover that, beginning in the late 1980s, Lawrence Johnson and other researchers had been applying a similar cell-sorting procedure to separate large numbers of X- and Y-sperms. In fact, the relatively small difference of about 3% in DNA content between the two sperm types is sufficient for effective separation. Initial work was conducted on domestic mammals such as rabbits, but by 1993 Johnson and reported results for pre-selecting sex of human babies. Fractions enriched for X-sperms yielded 82% positive results, while 75% enriched for Y-sperms gave a positive signal. Subsequently, in a 1999 review paper co-authored by Glenn Welch, Johnson reported that technical refinements had augmented the speed and reliability of sperm sorting. A success rate exceeding 90% had been obtained with several domestic mammal species and humans.
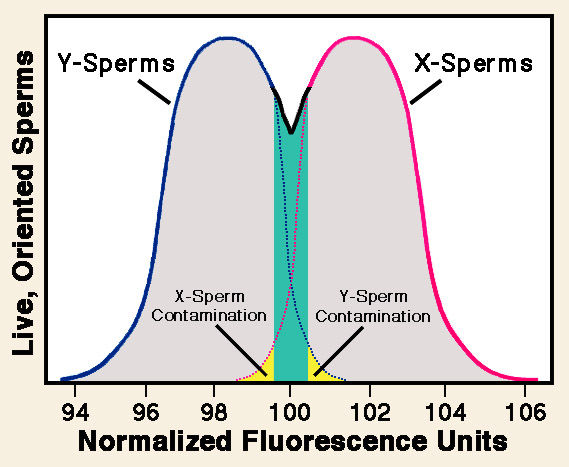
More recently, in 2008, Duane Garner and George Seidel provided a historical overview of commercialized use of sex-sorted sperms with cattle. Following significant improvements in the efficiency of flow sorting of bull sperms and successful cryopreservation, worldwide distribution rapidly developed. Financial advantages of sex pre-selection more than outweigh the relatively high cost of sex-sorted sperms, notably in the dairy industry, which needs bulls only for breeding.
Successful, high-precision separation of X- and Y-sperms in large numbers by flow sorting has paved the way for new research into possible differences. An excellent example is a 1998 paper by Linda Penfold and colleagues, examining motility of X- and Y-sperms from bull semen. They measured swimming speed, mean angular displacement, linearity, straightness of path, tail-beat frequency and extent of lateral head movements. X- and Y-sperms did show significant differences for mean angular displacement, linearity and straightness of path. By contrast, no significant differences were found for swimming speed, tail-beat frequency or lateral head movements. So the assumption that Y-sperms swim faster than X-sperms — shared by Shettles, Ericsson and many others — was not confirmed.
Sperm sorting by female mammals?
As it has at last proved possible to separate X- from Y-sperms, it is time to re-examine the possibility of selection in the genital tract of female mammals. Carmen Almiñana and colleagues reported one step in this direction, using state-of-the-art techniques, in a 2014 paper describing research on pigs, designed to determine whether X- and Y-sperms may be distinguished in the oviduct.
The researchers exploited the fact that female mammals have two oviducts. Using a laparoscope for guidance, a population of X-sperms was inseminated into one oviduct and a population of Y-sperms inseminated into the other. It emerged that genetic activity of the oviduct in response to sperms differed significantly according to whether they were carrying X- or Y-chromosomes. Moreover, local immune responses specific to each sperm type were evoked within the oviduct. It is hence clear that oviducts respond differently to X- and Y-sperms, providing a possible basis for a sex-biasing mechanism under female control.
Implications
After decades of controversy and conflicting reports, it is now possible to separate X- and Y-sperms with considerable (although not perfect) precision. Going forward, this should be a considerable boon to research as well as enabling justifiable interventions to pre-select the sex of human babies. Let it be noted that sex pre-selection may be needed for sound medical reasons. Some inherited conditions are linked to genes located on the X-chromosome (e.g. haemophilia, hydrocephalus, Duchenne muscular dystrophy). These conditions mainly affect boys, so it is safer to have girls. It is also recognized that pre-selection of a baby’s sex may be desirable in order to achieve balance within families. But such justifications lie on a slippery slope, and careful monitoring will be necessary to forestall any future drift towards an excess of one sex.
In closing, I feel obliged to comment that research on pigs conducted by one of the authors cited here has been blocked by an unfortunate development. A major company owns patents protecting the sperm separation technology, and for some reason now prohibits users from conducting research using the separated X- and Y-sperms. It is a sad day when private companies that have benefited enormously from publicly funded and freely available scientific findings stand in the way of continued research!
References
Almiñana, C., Caballero, I., Heath, P.R., Maleki-Dizaji, S., Parrilla, I., Cuello, C., Gil, M.A., Vazquez, J.L., Vazquez, J.M., Roca, J., Martinez, E.A., Holt, W.V. & Fazeli, A. (2014) The battle of the sexes starts in the oviduct: modulation of oviductal transcriptome by X and Y-bearing spermatozoa. BMC Genomics 15,293:1-11.
Carson, S.A. (1988) Sex selection: the ultimate in family planning. Fertility & Sterility 50:16-19.
Ericsson, R.J., Langevin, C.N. & Nishino, M. (1973) Isolation of fractions rich in human Y sperm. Nature 246:421-424.
Evans, J.M., Douglas, T.A. & Renton, J.P. (1975) An attempt to separate fractions rich in human Y sperm. Nature 253:352-354.
Garner, D.L. & Seidel, G.E. (2008) History of commercializing sexed semen for cattle. Theriogenology 69:886-895.
Hossain, A.M., Barik, S. & Kulkarni, P.M. (2001) Lack of significant morphological differences between human X and Y spermatozoa and their precursor cells (spermatids) exposed to different prehybridization treatments. Journal of Andrology 22:119-123.
Johnson, L.A., Flook, J.P. & Hawk, H.W. (1989) Sex preselection in rabbits: live births from X and Y sperm separated by DNA and cell sorting. Biology of Reproduction 41:199-203.
Johnson, L.A. & Welch, G.R. (1999) Sex preselection: High-speed flow cytometric sorting of X and Y sperm for maximum efficiency. Theriogenology 52:1323-1341.
Johnson, L.A., Welch, G.R., Keyvanfar, K., Dorfmann, A., Fugger, E.F. & Schulman, J.D. (1993) Gender preselection in humans? Flow cytometric separation of X and Y spermatozoa for the prevention of X-linked diseases. Human Reproduction 8:1773-1739.
Lien, S., Szyda, J., Leeflang, E.P., Hubert, R., Zhang, L., Schmitt, K. & Arnheim, N. (2002) Single-sperm typing. Current Protocols in Human Genetics 32:1.6.1-1.6.18.
Penfold, L.M., Holt, C., Holt, W.V., Welch, D.G., Cran, D.G. & Johnson, L.A. (1998) Comparative motility of X and Y chromosome-bearing bovine sperm separated on the basis of DNA content by flow sorting. Molecular Reproduction & Development 50:323-327.
Ross, A., Robinson, J.A. & Evans, H.J. (1975) Failure to confirm separation of X- and Y-bearing sperm using BSA gradients. Nature 253:354-355.
Seidel, G.E. & Garner, D.L. (2002) Current status of sexing mammalian spermatozoa. Reproduction 124:733-743.
Shettles, L.B. (1960) Nuclear morphology of human spermatozoa. Nature 186:648-649.
Shettles, L.B. & Rorvik, D.M. (2011) How to Choose the Sex of Your Baby: The Method Best Supported by Scientific Evidence. (Sixth edition; Fully Revised and Updated). New York: Crown Publishing Group.




