Testosterone
A Sperm’s Obstacle Course to the Egg
Men and women have quite different tactics for sperm numbers
Posted September 6, 2017

Some men produce too many sperms. That little-mentioned fact was the focus of my previous blog post. (See Why Too Many Sperms Spoil the Egg, posted on August 11, 2017.) With too many sperms, an unusually dense cloud surrounds the egg and more than one sperm may penetrate (polyspermy). In most human cases, two sperms fertilize an egg, yielding an embryo with an extra set chromosomes in addition to the normal pair from the father and mother (triploid condition). The additional chromosome set inevitably has catastrophic effects, with loss of the fetus or death of the infant within hours of birth. Some kind of chromosomal abnormality is present in about half of all miscarriages, and a quarter of those abnormalities involve extra chromosome sets.
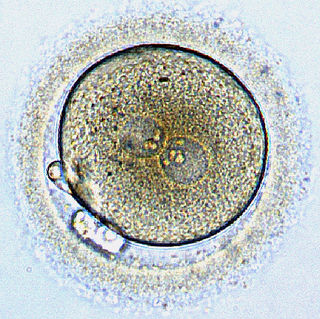
The sperm’s odyssey
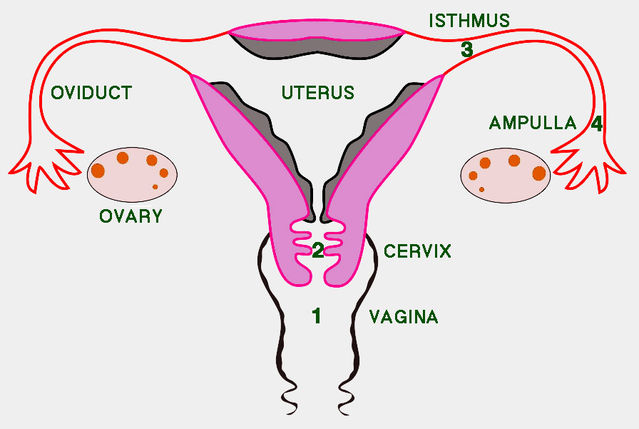
Although ejaculates of male mammals typically contain vast numbers of sperms — around 250 million on average in humans — surprisingly few usually get anywhere near an egg. Indeed, the reproductive tract of female mammals is seemingly specially adapted to minimize the number of sperms reaching the upper stretches of the oviduct (the tube along which the egg travels to reach the womb).
A 2006 paper by Susan Suarez and Allan Pacey expertly reviewed the odyssey facing sperms passing up the female reproductive tract. For starters, only part of the ejaculate escapes from the hostile acidity of the vagina into the neck of the womb (cervix). Then, as sperms migrate up the cervix, mucus strands filter out any that have abnormal shapes or swim too slowly. When the cervical barrier is bypassed by injecting semen directly into the womb (intrauterine insemination — IUI), pregnancy success levels off above 20 million sperms. This suggests that only 10% of sperms in a natural ejaculate reach the womb. Once sperms enter the womb, muscular contractions assist their passage to the oviduct. Only a few thousand sperms enter the relatively congenial environment of the oviduct. Its lower end, the isthmus, serves as a reservoir where sperms bind to the oviduct lining and are then released in a staggered fashion. After release, sperms undergo capacitation and become hyperactive, which enables them to travel to the upper end of the oviduct (ampulla), where fertilization occurs. The outcome of all obstacles encountered is that only a hundred or so sperms are typically present in the ampulla at any one time. Progressive reduction in sperm numbers between insemination and fertilization undoubtedly serves to reduce the risk of polyspermy.
Among many other suggestions, rampant speculation surrounding possible functions of the human orgasm spawned the hypothesis that it represents an adaptation to facilitate transportation of sperms toward the egg. It is known that orgasm is associated with enhanced release of the hormone oxytocin, which could potentially trigger active sperm transport. However, as Roy Levin noted in 2011, this hypothesis completely ignores the fact that avoiding polyspermy actually requires delicately balanced control of sperm transit through the female tract. Following insemination, the primary challenge for the female reproductive tract is to achieve a staged reduction of sperm numbers, not to speed transit of sperms towards the egg.
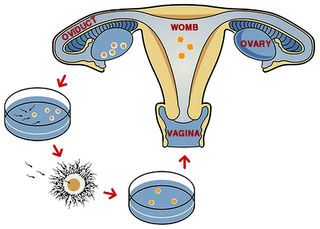
Lessons from in vitro fertilization
The advent of test-tube babies in 1978 opened up new possibilities for examining fertilization of the human egg, while also introducing the possibility that errors might arise because of inappropriate sperm densities. Yet this was not seen as a problem when in vitro fertilization (IVF) was first developed. In 1981, IVF pioneer Robert Edwards provided one of the first comments on possible polyspermy. He reported from initial work that a fetus that miscarried in the twelfth week of pregnancy had been found to be triploid. Although Patricia Jacobs and colleagues had previously reported in 1978 results from a major survey showing that triploidy is relatively common (1-3%) in human conception, Edwards stated that this chromosomal anomaly “may not be serious quantitatively” because the vast majority of eggs are fertilized by a single sperm. Admittedly, the frequency that Jacobs and colleagues reported was for natural conceptions. In 1981, no comparable information was available for eggs exposed to an unnatural sperm density in vitro.
In fact, in a 1981 paper Ian Craft and colleagues explicitly discussed sperm numbers in relation to IVF. They noted that the number of sperms surrounding an egg during natural conception was unknown and that the ideal number of sperms for in vitro fertilization had not been evaluated. Edwards and colleagues reportedly used between 100,000 and a million sperms, whereas the Craft team achieved fertilization with only 10,000 motile sperms in the culture medium surrounding the egg. They predicted that far lower numbers would eventually prove to be sufficient, “thus reducing the risk of polyspermic fertilization”.
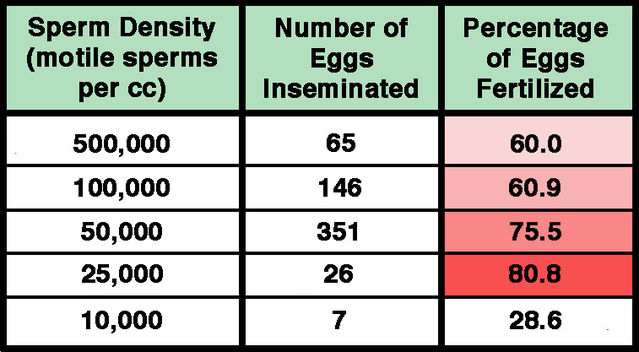
Subsequently, in 1984, Don Wolf and colleagues reported in more detail on relationships between sperm concentration and in vitro fertilization of human eggs. Fertilization success was actually found to decrease with increasing sperm numbers over the range of 25,000 to 500,000, with maximal fertilization of 80.8% at the lowest density. By contrast, the degree of polyspermic fertilization was directly related to sperm concentration, increasing from zero at less than 25,000 sperms/cc to 5.5% at 500,000 sperms/cc. Wolf and colleagues emphasized that concentrations of between half a million and a million sperms per egg were remarkably high compared with the hundred or so estimated to be present at the fertilization site in natural conception. A 1985 paper by Hans van der Ven and colleagues reinforced the findings reported by the Wolf team. Since the 1980s, relatively little has been published regarding optimal sperm numbers for IVF. As a rule, relatively low sperm densities are now used, and in 2013 Ping Xia reported that under these conditions approximately 7% of fertilized eggs are polyspermic. In modern IVF procedures, routine examination eliminates any such cases before transfer to the womb.
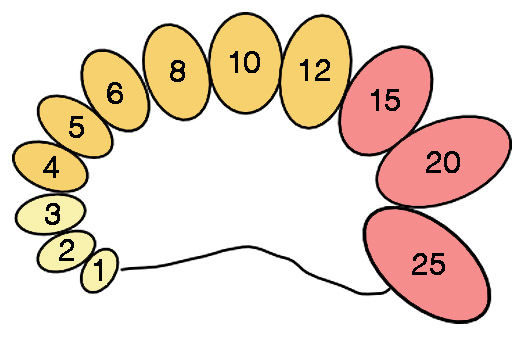
Diagram of an orchidometer for assessment of testis volume. Numbers indicate volume in ccs. Sizes 1–3 ccs (yellow) are typically found before puberty, sizes 4–12 ccs (orange) generally occur during puberty, and sizes 15–25 ccs (red) are usually found in adults.
Testis size, testosterone and sperm counts
Numerous studies have shown that testis size, testosterone levels and sperm production are all connected together in a functional network. Testis volume is often estimated through calculation from maximum length and width measured with calipers. In a 2004 paper, for instance, Leigh Simmons and colleagues reported a strong correlation between testis size and sperm counts calculated from linear measurements in a study of student volunteers. In many studies conducted by medical professionals, however, testis volume is determined by palpation accompanied by comparison with a standard set of ovoid models originally designed by Andrea Prader (1966). This device, known as an orchidodometer, is composed of twelve wooden or plastic ovoids with volumes of 1, 2, 3, 4, 5, 6, 8, 10, 12, 15, 20, and 25 ccs, respectively. Pediatricians regularly use orchidometers (“the urologist’s stethoscope”) to study individual development. On average, testes grow very little from birth up to the eleventh year (1-3 ccs), after which they begin to increase in size to reach about 12 ccs during puberty. Subsequent growth is very rapid, and the transition to the adult condition (typical range: 15-25 ccs) takes just three years.
With high testosterone levels, large testes and over-the-top sperm counts, some men may be “hypermasculine”. Confirming the suspicions of many women, it may truly be said that men may suffer from testosterone poisoning. A major downside of producing unusually large numbers of sperms is that it increases the potential for polyspermy with consequent disruption of fetal development. Presumably, natural selection generally operates to maintain sperm production at an optimal level reflecting a compromise between maximizing the probability of successful fertilization and minimizing the risk of polyspermy. And the female reproductive tract is evidently adapted for radical reduction of sperm numbers in a staged fashion.
References
Bujan, L., Mieusset, R., Mansat, A., Moatti, J.P., Mondinat, C. & Pontonnier, F. (1989) Testicular size in infertile men: relationship to semen characteristics and hormonal blood levels. British Journal of Urology 64:632-637.
Craft, I., McLeod, F., Bernard, A., Green, S. & Twigg, H. (1981) Sperm numbers and in-vitro fertilisation. Lancet 318:1165-1166.
Edwards, R.G. (1981) Test-tube babies, 1981. Nature 293:253-256.
Jacobs, P.A., Angell, R.R., Buchanan, I.M., Hassold, T.J., Matsuyama, A.M. & Manuel, B. (1978) The origin of human triploids. Annals of Human Genetics 42:49-57.
Levin, R.J. (2011) The human female orgasm: a critical evaluation of its proposed reproductive functions. Sexual & Relationship Therapy 26:301-314.
Pasqualotto, E.B., Daitch, J.A., Hendin, B.N., Falcone, T., Thomas, A.J., Nelson, D.R. & Agarwal.A. (1999) Relationship of total motile sperm count and percentage motile sperm to successful pregnancy rates following intrauterine insemination. Journal of Assisted Reproduction & Genetics 16:476-482.
Prader, A. (1966) Testicular size: Assessment and clinical importance. Triangle 7:240-243.
Simmons, L.W., Firman, L.C., Rhodes, G. & Peters, M. (2004) Human sperm competition: testis size, sperm production and rates of extrapair copulations. Animal Behaviour 68:297-302.
Suarez, S.S. & Pacey, A.A. (2006) Sperm transport in the female reproductive tract. Human Reproduction Update 12:23-37.
van der Ven, H.H., Al-Hasani, S., Diedrich, K., Hamerich, U., Lehmann, F. & Krebs, D. (1985) Polyspermy in in vitro fertilization of human oocytes: frequency and possible causes. Annals of the New York Academy of Sciences 442:88-95.
Wolf, D.P., Byrd, W., Dandekar, P. & Quigley, M.M. (1984) Sperm concentration and the fertilization of human eggs in vitro. Biology of Reproduction 31:837-848.
Xia, P. (2013) Biology of polyspermy in IVF and its clinical indication. Current Obstetrics and Gynecology Reports 2:226-231.




